Wei Liu
Tel: +86-0531-66659572; Fax: +86-0531-66658156;
E-mail: wheiliu@163.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 5
Page No: 753-759
Wei Liu
Tel: +86-0531-66659572; Fax: +86-0531-66658156;
E-mail: wheiliu@163.com
Shigang Zheng, Wei Liu*, Zhen Li, Qingguo Wang, Jiaowen Pan, and Fangyin Yao
Bio-Tech Research Center, Shandong Academy of Agricultural Sciences; Key Laboratory of Genetic Improvement, Ecology and Physiology of Crops, Shandong Province, Jinan 250100, China
Hong Yang(yanghong@mail.kib.ac.cn)
Chaolei Liu(liuchaolei505@163.com)
Ahmad A Omar(omar71@ufl.edu)
Sayed AbdulAzeez(asayed@iau.edu.sa)
Wei Liu, Investigation of Blood Lipid-decreasing Effect of Resveratrol and Genetic-modified Rice on Mice(2019)SDRP Journal of Food Science & Technology 4(5)
Many resveratrol (Res) enriched genetic-modified (GM) fruits or crops were obtained through biotechnology. In this paper, the blood lipid-decreasing effects of exogenously supplemented Res and endogenously accumulated Res in rice grains were evaluated. The accumulation of triglyceride (TG) and cholesterol (CHO) induced by egg yolk emulsion in mice blood was suppressed by fenofibrate (P < 0.01) and purified Res (P < 0.05). However, the GM rice modified by peanut resveratrol synthase gene 1 (PNRS1) did not show a significant variance to wild-type (WT) rice. The possible reason might be that the content of Res in GM rice was hard to meet the usually used concentration of purified compounds. So, this fast evaluating method was limited to investigate the health care functions of some GM foods enriched active components in trace. Further attempts will be done to develop health functional rice of Res by genetic engineering to meet the growing needs of consumers.
Keywords: Oryza sativa, hyperlipidemia, triglyceride, cholesterol , health functional food
Resveratrol, as a phytoalexin found in plant, has shown multi-functional effects on animals and human beings. The lifespan extension capacity of Res was reported in Yeast [1], Drosophila [2], C. elegans [3], Fly [4], Honeybee [5], and Mice [6]. Plenty of researches also reported the beneficial effects of Res in cancer prevention in several cancer cell lines [7,8], inflammation inhibition [9], and cardiovascular protection [10,11] in the aspects of health care, preventing and curing disease.
Res was shown the medicinal effects of leading to reducing lipid synthesis, increasing rates of fatty acid oxidation and preventing alcoholic liver steatosis [12]. Res has also been shown to attenuate high blood pressure and prevent cardiac hypertrophy in rats and mice [11]. When treated with procyanidin extracted from grape seed, the lipid and CHO metabolisms were altered between standard diet and high-fat diet fed hamsters [13]. To use Res in health functional foods [14], testing of GM products has also been performed on experimental animals, such as rats and mice. The rice of Cry1Ab protein contained mfb-MH86 was used to feed rats, and the results showed it is as safe and nutritious as the effects of non-GM rice [15]. The hearts of rats fed with Res modified tomato showed better cardiac performance, reduced myocardial infarct size and decreased number of apoptotic cardiomyocytes [10]. There was also research reporting that Res-enriched rice has more potent anti-metabolic syndrome activity than Res itself in mice by feeding high-fat diet [16].
For biosynthesis of Res, resveratrol synthase (RS) gene played a key role. It catalyzes coumaryl Co-A and malonyl Co-A to synthesize Res. Most of the plants contained the two substrates. But many food crops cannot synthesize Res because of the lacking RS gene [16]. In the previous study, a RS gene named PNRS1 (GenBank: FM955393) was cloned from Arachis hypogaea [17]. The RS enzyme activity of PNRS1 was confirmed in Escherichia Coli [18]. The binary vector of pCA1300-Ubi-PNRS1 was also conducted and used for transforming PNRS1 into Oryza sativa [17]. Molecular identification and secondary metabolic detecting indicated the successful producing of endogenous Res enriched GM rice.
In this study, the grains of the Res enriched GM rice were used to feed the mice to evaluate their health care effects. Together with the blood lipid decreasing drug fenofibrate, the benefits of exogenously supplemented Res and endogenously accumulated Res to blood lipid decreasing were investigated in hyperlipidemia modelled mice. This research will provide reference for producing and providing better candidate resources of health functional food and point out the direction of optimization to further meet people's growing requirements of health care needs.
GM rice
Res enriched rice was developed by transforming peanut RS gene of PNRS1 into Oryza sativa cv. shengdao13 [17, 18]. The transgenic lines were cultivated for purification in the experimental fields. And a stable line L3 showing highest concentration of Res was selected for this experiment. Finally, about 5 kg seeds of wild-type and transgenic rice L3 were acquired for this experiment. Pretreatment of seeds with removing the seed coat and grounding the polished grains to fine powder were also performed.
Animals
Six-week-old Kunming mice (KM) of half male and half female were purchased from animal center of Shandong Lukang Pharmaceutical Co. (China). The male and female mice were caged individually. The mice were permitted access to food and water ad libitum. Specific pathogen free (SPF) folder of mice (Keaoxieli Inc., China) was the control diet. The mice were cultured in the animal room of Institute of Materia Medica, Shandong Academy of Chinese Medicine, under a 12 h light/12 h dark cycle at a temperature of 20-26℃ and humidity of 40-70%. Sixty KM mice were randomly divided into six groups, including normal control group (CK), negative control group (CK-), positive control group (CK+), external resveratrol treated group (RES), GM rice fed group (GM) and WT rice fed group (WT). Each group contained five male and five female mice.
Detection of trans-resveratrol
The content of Res in GM rice seeds was detected by high performance liquid chromatography (HPLC). The parameters of HPLC analysis were referred to Zheng et al. [18]. The trans-resveratrol (3,40,5-trihydroxy-trans-stilbene 99% GC; Sigma-Aldrich, St. Louis, MO) was employed as the standard sample. The amounts of Res were quantified by the corresponded peak areas.
Induction of the hyperlipidemia model
The treatments of each group were listed in Table 1. Fenofibrate (Laboratoires Fournier S. A., France) and trans-resveratrol standard (3,4′,5-trihydroxy-trans- stilbene, 99% GC; Sigma-Aldrich, St. Louis, MO) were supplemented to grain powder of WT rice to a final concentration of 1 g/kg. The rice grain powder was suspended with pure water at a final concentration of 0.5 g/ml. And 1 ml of this homogenate was used as an enema for each mouse twice per day. After two weeks of cultivation, hyperlipidemia models were induced by intraperitoneal injection of 75% hen egg yolk emulsion [19] in the mice of CK-, CK+, RES, GM, and WT group. The mice of CK group were injected with normal saline. Then, all the mice were fed for 12 h as usual, and then were fasted for 4 hours before blood sampling.
Determination of triglyceride and cholesterol
To measure the blood lipid levels, the eyeballs of mice of each group were extracted and blood was drawn from the tail, and the serum was separated by centrifuging at 13,000 rpm for 10 min and immediately stored at -20℃ for further analysis. The levels of total TG and CHO in the serum were quantitatively determined by using an automatic biochemical analyzer (Hitachi 7180E; Japan) with reagents from Mike Biological Technologies Inc. (China).
Statistical analyses
All the data were recorded in Microsoft office excel 2010 for statistical analysis. The data was presented as mean ± standard deviation (SD). Student t-test was used to evaluate the variance and significance between each two of the experimental groups. The data of p value less than 0.05 was labeled.
Table 1. The feeding experiments on the mice of each group.
|
Groups |
Number of mice |
Diet |
Supplements (1 g/d) |
Additive (1 g/kg) |
Culture (weeks) |
Injection (0.025 ml/g) |
Intake of Res or Drug (mg/kg/d) |
|
CK |
10 |
SPF |
NO1 |
NO |
4 |
normal saline |
0 |
|
CK- |
10 |
SPF |
NO |
NO |
4 |
egg yolk emulsion |
0 |
|
CK+ |
10 |
SPF |
WT rice |
fenofibrate |
4 |
egg yolk emulsion |
50 |
|
RES |
10 |
SPF |
WT rice |
Res |
4 |
egg yolk emulsion |
50 |
|
GM |
10 |
SPF |
GM rice |
NO |
4 |
egg yolk emulsion |
0.05 |
|
WT |
10 |
SPF |
NO |
NO |
4 |
egg yolk emulsion |
0 |
1 NO nothing was supplemented or added.
Endogenous Res enriched GM rice foods
After HPLC detection, the concentration of trans-resveratrol was about 3 µg/g dry weight (DW) in the selected transgenic rice seeds [Fig. 1]. More, the Res content in GM rice polished grains was only 1 µg/g DW. While almost none of trans-resveratrol was identified in WT rice seeds and polished grains. This means that the trans-resveratrol was newly accumulated into GM rice. And the polished grains of GM rice were used as endogenous Res enriched functional foods for further analysis.
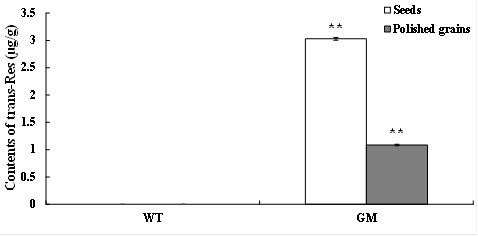
Fig. 1. The contents of trans-resveratrol in GM rice foods. trans-Res trans-resveratrol, WT wild-type rice, GM genetic-modified rice. ** indicates p < 0.01 compared to any one of the groups. The basic data has been reported by Zheng et al. previously (18).
Successful construction of hyperlipidemia model in mice
The levels of TG and CHO were used to appraise the accumulation of blood lipid [20]. So, the concentration of them was measured in serum of mice obtained from each experimental group. Almost all the mice were survived through the experiment, except that there was a death mouse in the CK+ and GM groups. The average levels of CHO and TG in each group were shown in Fig. 2A and Fig. 2B, respectively. The contents of TG and CHO in CK group were 2.412 ± 0.252 mmol/L and 1.004 ± 0.233 mmol/L, respectively. While there are 9.265 ± 1.873 mmol/L of CHO and 13.985 ± 3.744 mmol/L of TG in CK- group. Both levels of TG and CHO in CK- group were significantly higher than those in CK group (p<0.01). This indicates that the hyperlipidemia model in mice was successfully induced by the egg yolk emulsion.
External purified Res showing blood lipid reducing effect
The contents of TG and CHO in CK+ group were 6.262 ± 1.199 mmol/L and 8.851 ± 2.829 mmol/L, respectively. They were significantly lower (p < 0.01) than those in CK- group. This means that the lipid-decreasing control of fenofibrate was constructed successfully. Then, the function of exogenously supplemented Res and endogenously enriched Res in rice grain can be analyzed. The contents of TG and CHO in RES group are 7.487 ± 1.641 mmol/L and 10.008 ± 3.473 mmol/L, respectively. They were significantly lower (P < 0.05) than those in CK- group. This reveals that exogenously supplemented Res plays a role in decreasing of blood lipids.
No significant blood lipid decreasing effect observed for GM rice
However, the contents of TG and CHO showed no significant differences existed among CK-, GM, and WT groups. There were 9.965 ± 2.543 mmol/L of TG and 14.827 ± 4.380 mmol/L of CHO in GM group, and there are 10.072 ± 2.889 mmol/L of TG and 15.632 ± 4.670 mmol/L of CHO in WT group. This suggests that GM rice grains modified by peanut RS gene PNRS1 do not meet a significant effect for blood lipid decreasing in this fast-evaluating experimental system.
Polyphenolic compounds are the important constituents of traditional health functional food, such as propolis [21], Red Wine [22], and Green tea [23]. The polyphenolic structure of Res also made it play a role in health care through involving in reactive oxygen species (ROS) related responses and regulating a series of ROS signal participated pathways [8,24,25]. To utilize this beneficial component, new type of health functional food was developed by genetic engineering. At present, various fruits, vegetables, and crops, such as in kiwifruits [26], apple [27], oilseed rape [28], pea [29], lettuce [30], tomato [10], potato [31], and rice [16,18] had been constructed the enrichment of Res through transgenic technology.
In this study, the purified Res showed a similar effect on blood lipid decreasing with fenofibrate in hyperlipidemia modelled mice (Fig. 2). The usually used supplementing dose of commercialized Res was 40 mg/kg to 320 mg/kg in previous researches [6, 11, 12]. And considered the dose of blood lipid decreasing drug fenofibrate, the final intake of purified Res was 50 mg/kg/d in this study. But the highest concentration of Res in the GM rice seeds was 3 µg/g, and the Res content in GM rice polished grains was only 1 µg/g [18]. This led to the final intake of Res in GM group was only 0.05 mg/kg/d (Table 1). Moreover, there was only 1.9 µg/g of Res in transgenic rice grain developed by Baek et al. [16]. And there was only 2.586 μg/g, 2.7 μg/g, 0.79–15.8 μg/g, and 56.4 μg/g of Res having been reported in transgenic grape [32], hops [33], tomato [10], and lettuce [30], respectively. So, there is still a long way to improve the contents of Res in GM products to reach an effective dose.
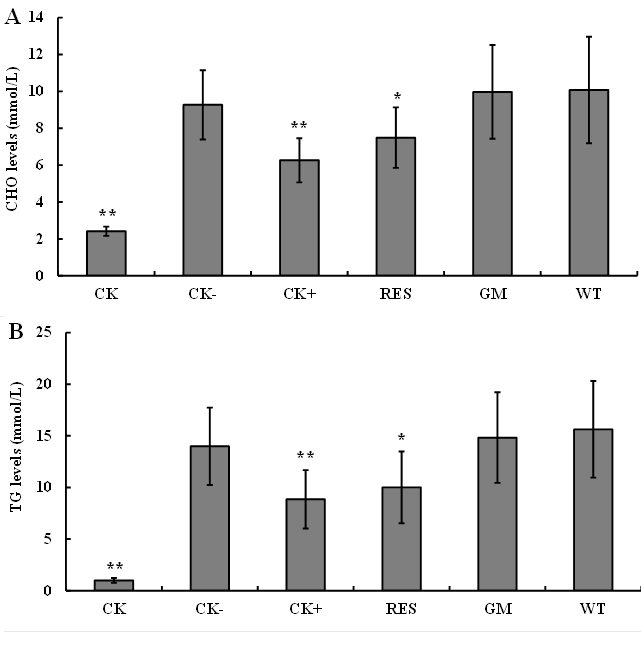
Fig. 2. Change of hyperlipidemia indexes in the mice with different treatments. A cholesterol (CHO) levels in the serum of mice; B triglyceride (TG) levels in the serum of mice. CK normal control group; CK- negative control group; CK+ positive control group treated by fenofibrate (50 mg/kg/d); RES external resveratrol (50 mg/kg/d) treated group; GM genetic-modified rice (0.15 mg/kg/d of Res) fed group; WT wild-type rice (0 mg/kg/d of Res) fed group (WT). Each group contained five male and five female mice Except the mice of CK group were injected with normal saline, the mice of other groups were intraperitoneally injected with 75% egg yolk emulsion to induce hyperlipidemia. Bars represent the mean values ± SD (n ≥ 9). ** indicates p < 0.01, * indicates p < 0.05 compared to CK- group.
The mice fed with GM rice grains did not show a significant difference to those fed with wild-type rice grains after hyperlipidemia modelling (Fig. 2). This is mainly because of that the endogenous accumulation of Res in GM rice modified by heterogenous RS gene is still very low [16, 18]. And it cannot meet the effective dose as exogenously supplementing experiment usually used. Furthermore, the fast method by determining the levels of TG and CHO to evaluate the blood lipid-decreasing effect might be useful for the commercialized drug or purified Res. But there was only low dose of efficacious constituents in some health functional foods. The health care function of them depends on a relatively long term to express the accumulated effects.
The expression and enrichment of Res in GM rice over-expressing RS gene still need to be further optimized and screened to meet the customer needs. As Res showed multiple health care functions, the Res enriched GM food will attract more attention and apply in various of fields soon. Certainly, the actual benefits of Res-enriched diet to health care still need to be deeply and intensively studied before commercialization.
This work was financially supported by Natural science foundation of Shandong province (ZR2019QC003), National key research and development program of China (2016YFD0100903-9), Agricultural scientific and technological innovation project of Shandong Academy of Agricultural Sciences(CXGC2016A02), Shandong agricultural of seed project (2017LZN029).
Agricultural scientific and technological innovation project of Shandong Academy of Agricultural Sciences (CXGC2018E13), China Agriculture Research System (CARS-06) and the Young Talents Training program of Shandong Academy of Agricultural Sciences (2015-2017, 2016-2018).
Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 425: 191-196 (2003)
View ArticleAntosh M, Whitaker R, Kroll A, Hosier S, Chang C, Bauer J, Cooper L, Neretti N, Helfand SL. Comparative transcriptional pathway bioinformatic analysis of dietary restriction, Sir2, p53 and resveratrol life span extension in Drosophila. Cell Cycle. 10: 904-911 (2011)
View ArticleViswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2,1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell. 9: 605-615 (2005)
View ArticleZou S, Carey JR, Liedo P, Ingram DK, Müller HG, Wang JL, Yao F, Yu B, Zhou A. The prolongevity effect of resveratrol depends on dietary composition and calorie intake in a tephritid fruit fly. Exp. Gerontol. 44: 472-476 (2009)
View ArticleRascón B, Hubbard BP, Sinclair DA, Amdam GV. The lifespan extention effects of resveratrol are conserved in the honey bee and may be driven by a mechanism related to caloric restriction. Aging. 4: 499-508 (2012)
View ArticlePearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, Jamieson HA, Zhang Y, Dunn SR, Sharma K, Pleshko N, Woollett LA, Csiszar A, Ikeno Y, Le Couteur D, Elliott PJ, Becker KG, Navas P, Ingram DK, Wolf NS, Ungvari Z, Sinclair DA, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8: 157-168 (2008)
View ArticleAmiri F, Zarnani AH, Zand H, Koohdani F, Jeddi-Tehrani M, Vafa M. Synergistic anti-proliferative effect of resveratrol and etoposide on human hepatocellular and colon cancer cell lines. Eur. J. Pharmacol. 718: 34-40 (2013)
View ArticleLin CY, Hsiao WC, Wright DE, Hsu CL, Lo YC, Hsu GW, Kao CF. Resveratrol activates the histone H2B ubiquitin ligase, RNF20, in MDA-MB-231 breast cancer cells. J. Funct. Foods. 5: 790-800 (2013)
View ArticleD'Introno A, Paradiso A, Scoditti E, D'Amico L, De Paolis A, Carluccio MA, Nicoletti I, DeGara L, Santino A, Giovinazzo G. Antioxidant and anti-inflammatory properties of tomato fruits synthesizing different amounts of stilbenes. Plant Biotechnol. J. 7: 422-429 (2009)
View ArticleNicoletti I, De Rossi A, Giovinazzo G, Corradini D. Identification and quantification of stilbenes in fruits of transgenic tomato plants (Lycopersicon esculentum Mill.) by reversed phase HPLC with photodiode array and mass spectrometry detection. J. Agric. Food Chem. 55: 3304-3311 (2007)
View ArticleDolinsky VW, Chakrabarti S, Pereira TJ, Oka T, Levasseur J, Beker D, Zordoky BN, Morton JS, Nagendran J, Lopaschuk GD, Davidge ST, Dyck JR. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta. 1832: 1723-1733 (2013)
View ArticleAjmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am. J. Physiol. Gastrointest Liver Physiol. 295: 833-842 (2008)
View ArticleCaimari A, Crescenti A, Puiggròs F, Boqué N, Arola L, Del Bas JM. The intake of a high-fat diet and grape seed procyanidins induces gene expression changes in peripheral blood mononuclear cells of hamsters: capturing alterations in lipid and cholesterol metabolisms. Genes Nutr. 10: 438-448 (2015)
View ArticleGoetzke B, Nitzko S, Spiller A. Consumption of organic and functional food. A matter of well-being and health? Appetite. 77: 94-103 (2014)
View ArticleSong H, He X, Zou S, Zhang T, Luo Y, Huang K, Zhu Z, Xu W. A 90-day subchronic feeding study of genetically modified rice expressing Cry1Ab protein in Sprague–Dawley rats. Transgenic Res. 24: 295-308 (2015)
View ArticleBaek SH, Shin WC, Ryu HS, Lee DW, Moon E, Seo CS, Hwang E, Lee HS, Ahn MH, Jeon Y, Kang HJ, Lee SW, Kim SY, D'Souza R, Kim HJ, Hong ST, Jeon JS. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PLoS One. 8: e57930 (2013)
View ArticleLi HQ, Liu X, Wang QG, Yao FY, Liu W. Construction of biosafe vectors of resveratrol synthase gene and rice genetic transformation. Acta Agriculturae Breali-Sinica. 26: 114-118 (2011)
View ArticleZheng S, Zhao S, Li Z, Wang Q, Yao F, Yang L, Pan J, Liu W. Evaluating the effect of expressing a peanut resveratrol synthase gene in rice. PLoS One. 10: e0136013 (2015)
View ArticleGao X, Jiang C, Xu J, Yanagita T, Xue C, Wang Y. Serum pharmacokinetics of choline, trimethylamine, and trimethylamine-N-oxide after oral gavage of phosphatidylcholines with different fatty acid compositions in mice. Biosci. Biotechnol. Biochem. 13: 1-7 (2016)
View ArticleLee HS, Nam Y, Chung YH, Kim HR, Park ES, Chung SJ, Kim JH, Sohn UD, Kim HC, Oh KW, Jeong JH. Beneficial effects of phosphatidylcholine on high-fat diet-induced obesity, hyperlipidemia and fatty liver in mice. Life Sci. 118: 7-14 (2014)
View ArticleWang K, Jin X, Chen Y, Song Z, Jiang X, Hu F, Conlon MA, Topping DL. Polyphenol-Rich Propolis Extracts Strengthen Intestinal Barrier Function by Activating AMPK and ERK Signaling. Nutrients. 8: E272 (2016)
View ArticleBaur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5: 493-506 (2006)
View ArticleGaur S, Agnihotri R. Green tea: a novel functional food for the oral health of older adults. Geriatr. Gerontol. Int. 14: 238-250 (2014)
View ArticleKing RE, Bomser JA, Min DB. Bioactivity of resveratrol. Compr. Rev. Food Sci. F. 5: 65-70 (2006)
View ArticleTang K, Zhan JC, Yang HR, Huang WD. Changes of resveratrol and antioxidant enzymes during UV induced plant defense response in peanut seedlings. J. Plant Physiol. 167: 95-102 (2010)
View ArticleKobayashi S, Ding CK, Nakamura Y, Nakajima I, Matsumoto R. Kiwifruits (Actinidia deliciosa) transformed with a Vitis stilbene synthase gene produce piceid (resveratrol-glucoside). Plant Cell Rep. 9: 901-910 (2000)
View ArticleSzankowski I, Briviba K, Fleschhut J, Schönherr J, Jacobsen HJ, Kiesecker H. Transformation of apple (Malus domestica Borkh.) with the stilbene synthase gene from grapevine (Vitis vinifera L.) and a PGIP gene from kiwi (Actinidia deliciosa). Plant Cell Rep. 22: 141-149 (2003)
View ArticleHüsken A, Baumert A, Milkowski C, Becker HC, Strack D, Möllers D. Resveratrol glucoside (Piceid) synthesis in seeds of transgenic oilseed rape (Brassica napus L.). Theor. Appl. Genet. 111: 1553-1562 (2005)
View ArticleRichter A, Jacobsen HJ, Kathen A, Lorenzo G, Briviba K, Hain R, Ramsay G, Kiesecker H. Transgenic peas (Pisum sativum) expressing polygalacturonase inhibiting protein from raspberry (Rubus idaeus) and stilbene synthase from grape (Vitis vinifera). Plant Cell Rep. 25: 1166-1173 (2006)
View ArticleLiu S, Hu Y, Wang X, Zhong J, Lin Z. High content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J. Agric. Food Chem. 2006 54: 8082-8085.
View ArticlePan LP, Yu SL, Chen CJ, Li H, Wu YL, Li HH, Cloning a peanut resveratrol synthase gene and its expression in purple sweet potato. Plant Cell Rep. 31: 121-131 (2012)
View ArticleFan C, Pu N, Wang X, Wang Y, Fang L, Xu W, Zhang J. Agrobacterium-mediated genetic transformation of grapevine (Vitis vinifera L.) with a novel stilbene synthase gene from Chinese wild Vitis pseudoreticulata. Plant Cell Tiss. Organ. Cult. 92: 197-206 (2008)
View ArticleSchwekendiek A, Spring O, Heyerick A, Pickel B, Pitsch NT, Peschke F, Keukeleire D, Weber G. Constitutive expression of a grapevine stilbene synthase gene in transgenic hop(Humulus lupulus L.) yields resveratrol and its derivatives in substantial quantities. J. Agric. Food Chem. 55: 7002-7009 (2007)
View Article