Jacek Rysz,
Department of Nephrology, Hypertension and Family Medicine, Medical University of Lodz, Zeromskiego 113, Lodz, Poland,
E-mail: jacek.rysz@umed.lodz.pl
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 5
Page No: 431-439
Jacek Rysz,
Department of Nephrology, Hypertension and Family Medicine, Medical University of Lodz, Zeromskiego 113, Lodz, Poland,
E-mail: jacek.rysz@umed.lodz.pl
Magdalena Plewka1, Jacek Rysz1, Krzysztof Kujawski1,2
1Department of Nephrology, Hypertension and Family Medicine, Medical University of Lodz, Zeromskiego 113, 90-549 Lodz, Poland
2Gastrointestinal Endoscopy Department, WAM Teaching Hospital of Lodz, Zeromskiego 113, 90-549 Lodz, Poland
Lu Ke L(kkb9832@163.com)
Jacek Rysz, Nutrition and malnutrition in chronic pancreatitis (2018)SDRP Journal of Food Science & Technology 3(5)
Patients with chronic pancreatitis are at risk of malnutrition and nutrient deficiencies. ddMalnutrition is a huge problem in population, especially in hospitalised patients. Routine assessment and regular monitoring of nutrition status is essential. It is associated with increased rates of morbidity and mortality in hospital patients and significantly increases healthcare costs. The pancreas is a major player in digestion. Normal pancreatic function ensures effective digestion and absorption of nutrients. Chronic pancreatitis refers to a syndrome of long-standing pancreatic injury and because of its role in digestion, chronic pancreatitis is responsible for malnutrition.
According to WHO definition, malnutrition refers to deficiencies, excesses or imbalances in a person’s intake of energy and/or nutrients. The term malnutrition covers 2 broad groups of conditions. One is ‘undernutrition’— which includes stunting (low height for age), wasting (low weight for height), underweight (low weight for age) and micronutrient deficiencies or insufficiencies (a lack of important vitamins and minerals). [1] Malnutrition is a common, under-recognised and undertreated problem facing patients and clinicians. It is both a cause and consequence of disease and exists in institutional care and the community. It concerns a very large population of developing countries, but it can also be a problem in developed countries, including Poland.
Generally about 20%-50% (depends on what criteria are taken) of all patients in hospital are found at risk of undernutrition. A large part of these patients are at nutritional risk when admitted to hospital and in the majority of these, undernutrition develops negatively during hospital stay. It is really important to prevent this undernutrition, because this can cause difficulties with treating main diseases that are responsible for admitting to hospital. [2]
There are several factors that can have influence on failure in nutrition.
Table 1. Factors contributing to malnutrition in acute care patients (published by Elsevier, 2007). [3]
|
Personal |
Organisational |
|
Age |
Failure to recognise malnutrition |
|
Apathy/depression |
Lack of nutritional screening or assessment |
|
Disease (e.g., cancer, diabetes, cardiac, gastrointestinal) |
Lack of nutritional training |
|
Inability to buy, cook or consume food |
Confusion regarding nutritional responsibility |
|
Inability to chew or swallow |
Failure to record height and weight |
|
Limited mobility |
Failure to record patient intake |
|
Sensory loss (taste, smell) |
Lack of adequate intake |
|
Treatment (ventilation, surgery, drain tubes) |
Lack of staff to assist with feeding |
|
Drug therapy |
Importance of nutrition unrecognised |
Most adult malnutrition is associated with disease and may arise due to:
Malnutrition, which is often overlooked by clinicians, is common and has wide-ranging effects on physiological function. It is associated with increased rates of morbidity and mortality in hospital patients and significantly increases healthcare costs. Implementation of a simple screening tool identifies patients at risk and allows appropriate treatment to be instituted; this can significantly improve clinical outcomes and reduce healthcare expenditure. Every doctor should know that proper nutritional care is essencial to good clinical practice.
There are several tools to identify patients with malnutrition. [3]
1.MUST is a simple, rapid only three-question tool to screen patients and has been proven to be reliable and valid. It aims to identify those at risk by incorporating:
It allows indicate whether nutrition intervention is necessary. Although is limited by the fact it has not been validated in children or renal patients.
2.The Mini Nutrition Assessment (MNA) was developed specifically for use among elderly patients (≥65 years) in hospitals and nursing homes. The original form considers: anthropometrical, medical, lifestyle, dietary and psychosocial factors in an 18 item assessment, using a points-based scoring system to determine if a patient is at risk of, or suffering from malnutrition.
2.Nutritional Risk Screening (NRS-2002) uses recent weight loss, decreased BMI and reduced dietary intake, combined with a subjective assessment of disease severity (based on increased nutrition requirements and/or metabolic stress), to generate a nutrition risk score.
3.The four item Short Nutrition Assessment Questionnaire (SNAQ) was developed to diagnose malnutrition in hospitalised patients and provides an indication for dietetic referrals as well as outlining a nutrition treatment plan. It has been validated for hospital inpatient and outpatient use, as well as residential patients and does not require calculation of BMI.
4.Subjective Global Assessment (SGA) as dr Khursheed Jeejeebhoy says „is a simple bedside method of assessing the risk of malnutrition and identifying those who would benefit from nutritional support. Its validity for this purpose has been demonstrated in a variety of conditions including surgical patients, those with cancer, on renal dialysis and in the ICU.”
SGA is one of the most commonly used nutrition assessment tools, and assesses nutrition status via completion of a questionnaire which includes data on weight change, dietary intake change, gastrointestinal symptoms, changes in functional capacity in relation to malnutrition as well as assessment of fat and muscle stores and the presence of oedema and ascites [4]. This tool allows for malnutrition diagnosis, and classifies patients as either: A—well-nourished; B—mildly/moderately malnourished; or C—severely malnourished.
SGA has been found to be an appealing method of assessing nutritional status, as its subjective nature allows clinicians to capture subtle patterns of change in clinical variables (e.g., weight loss patterns rather than absolute weight loss). A high degree of inter-rater reproducibility has been shown for SGA, with 91% of surgical patients classified by SGA having two clinicians agreeing on SGA classification [4].
Consequences of malnutrition
It can decrease muscle function due to depletion of fat and muscle mass. Muscle function declines before changes in muscle mass occur, suggesting that altered nutrient intake has an important impact independent of the effects on muscle mass. If, dietary intake is insufficient to meet requirements over a more prolonged period of time the body draws on functional reserves in tissues such as muscle, adipose tissue and bone leading to changes in body composition. With time, there are direct consequences for tissue function, leading to loss of functional capacity and a brittle, but stable, metabolic state. [6,20]
Reduction in cardiac muscle mass is recognised in malnourished individuals. The resulting decrease in cardiac output has a corresponding impact on renal function by reducing renal perfusion and glomerular filtration rate. Micronutrient and electrolyte deficiencies (eg thiamine) may also affect cardiac function, particularly during refeeding. Poor diaphragmatic and respiratory muscle function reduces cough pressure and expectoration of secretions, delaying recovery from respiratory tract infections.
Adequate nutrition is important for preserving GI function: chronic malnutrition results in changes in pancreatic exocrine function, intestinal blood flow, villous architecture and intestinal permeability. The colon loses its ability to reabsorb water and electrolytes, and secretion of ions and fluid occurs in the small and large bowel. This may result in diarrhoea, which is associated with a high mortality rate in severely malnourished patients.
Immune function is also affected, increasing the risk of infection due to impaired cell-mediated immunity and cytokine, complement and phagocyte function. Delayed wound healing is also well described in malnourished surgical patients.
In addition to these physical consequences, malnutrition also results in psychosocial effects such as apathy, depression, anxiety and self-neglect. [20]
The consequences of malnutrition on physiological function have an important impact on clinical outcome. It is observed that patients who were starved or underweight have a higher incidence of postoperative complications and mortality. A large number of studies have subsequently supported this original observation. Malnourished surgical patients have complication and mortality rates three to four times higher than normally nourished patients, with longer hospital admissions, incurring up to 50% greater costs. Similar findings have also been described in medical patients, particularly the elderly. It is often difficult to separate the deleterious effects of malnutrition from the underlying disease process itself, especially because each can be a cause and/or consequence of the other. However, there is clear evidence that nutrition support significantly improves outcomes in these patients; it is therefore vital that malnutrition is identified through screening.[20]
Pancreas and its function
The pancreas is a gland which releases digestive enzymes and hormones. The pancreas has two main functions. The exocrine cells produce digestive enzymes to assist in digestion and the endocrine cells produce hormones to control metabolism. Pancreatic enzymes produced by acinar cells help to digest proteins, carbohydrates and fats. Some of these digestive enzymes include: pancreatic proteases (trypsin and chymotrypsin), pancreatic amylase and lipase. [21]
The endocrine cells of the pancreas produce hormones that control certain metabolic functions, including blood sugar regulation and digestion. Some of the hormones produced by the islets of Langerhans cells include: insulin, glucagon and gastrin
The production and release of pancreatic hormones and enzymes are regulated by the peripheral nervous system and gastrointestinal system hormones. Neurons of the peripheral nervous system either stimulate or inhibit the release of hormones and digestive enzymes based on environmental conditions.
Due to its role in digestion and its function as an endocrine organ, damage to the pancreas can have serious consequences. [11,12]
Chronic pancreatitis
Chronic pancreatitis refers to a syndrome of long-standing pancreatic injury. Histologic changes from the normal pancreatic architecture include irregular fibrosis, acinar cell loss, islet cell loss and inflammatory cell infiltrates. Histologic changes in the organ cause progressive loss of function and clinically important pain. It is characterised by the loss of exocrine and endocrine pancreatic parenchyma, irregular fibrosis, cellular infiltration, and ductal abnormalities. The clinical presentation of chronic pancreatitis is to a large extent depending on the stage of the disease, with a wide range of severity of symptoms and the natural history. [13,21]
Chronic pancreatitis is usually classified according to aetiology into alcoholic pancreatitis, biliary pancreatitis, chronic pancreatitis due to less common aetiologies (hereditary, hyperparathyroidism, etc) and idiopathic pancreatitis.
It can be divided into three stages: Early stage (stage A) is dominated by recurrent clinical acute pancreatitis and no complications, intermediate (stage B) by more constant pain and local complications from pseudocysts, pancreatic calcification and obstruction of adjacent organs and the end stage (stage C) by exocrine and/or endocrine insufficiency.
Epigastric pain, often radiating to the spine or left upper quadrant and correlated to intake of food, is the cardinal symptom in chronic pancreatitis and occurs in 80-90% of patients. The patophysiology of pain is multifactorial and complex and still ill-defined. Local complications such as pseudocysts or obstructive cholestasis seem to be the most common causes of persistent pain causing an increased pressure within the pancreatic duct or parenchyma. Drainage of the pancreatic duct can lead to immediate pressure and pain relief, but there are no intervations that provide complete pain relief in all patients. Permanent discontinuation of alcohol is an important factor influencing pain in alcoholic chronic pancreatitis. [21]
Chronic pancreatitis is responsible for malnutrition in patients
The pancreas is a major player in digestion. Normal pancreatic function ensures effective digestion and absorption of nutrients. Clinical exocrine pancreatic insufficiency occurs when secretions of the pancreas do not maintain normal digestive function. Pancreatic exocrine insufficiency (PEI) is one of the long-term consequences of chronic pancreatitis. Majority of patients with PEI were undiagnosed or undertreated. Inadequately treated or subclinical severe PEI causes malnutrition.
The pancreas has a large functional reserve and clinically evident PEI occurs only when 90% of the function is lost and the secretion of pancreatic enzymes is less than 10% of normal, which results in lipid malabsorption, steatorrhea (frothy, foul smelling, buoyant stools), weight loss, abdominal discomfort, and abdominal swelling are the common presenting symptoms and are related to the inadequate lipid digestion.
Consequences of abnormal lipid digestion lead to malnutrition, with malabsorption of lipid-soluble vitamins (A, D, E, K), depleted micronutrients, and decreased circulating lipoproteins.
Deficiencies in these vitamins and nutrients may lead to tetany, glossitis, cheilosis, and in a more progressive stage, to peripheral neuropathy. In addition, patients with PEI are at risk of developing significant bone loss. Because these patients have decreased serum levels of vitamin D metabolites and a low bone mass, it seems reasonable to consider a bone density scan to check for signs of osteoporosis. Several studies have reported malabsorption of zinc in advanced CP, but the mechanism of this phenomenon remains unclear. Folate deficiencies, although rare, have been reported because folate forms insoluble complexes with pancreatic extracts.
There seems to be an increased risk for cardiovascular events in EPI, independent of life style factors.
In chronic pancreatitis, the progression to gland failure depends on the underlying cause of the disease. Patients with alcoholic pancreatitis for instance, develop exocrine insufficiency after a median of ten years while in patients with idiopathic CP this may take up to 20 to 25 years to occur. Furthermore, in autoimmune pancreatitis, EPI is one of the presenting symptoms in over 75% of patients. [9]
Table 2. Etiologies of exocrine pancreatic insufficiency. [10]
|
Mechanism |
Etiology |
|
Decreased lipase production and delivery, increase lipase destruction |
Chronic pancreatitis, cystic fibrosis, diabetes |
|
Pancreatic duct obstruction |
Periampullary tumor, pancreatic head cancer, IPMN, benign tumors |
|
Decreased endogenous lipase stimulation and production |
Celiac disease, Crohn’s disease, Shwachman–Diamond syndrome |
|
Motility disorders (decrease contact time, interaction with chyme, decrease stimulation of pancreatic enzymes) |
Gastrectomy, gastric bypass, extensive small bowel resection |
The treatment of pancreatic exocrine insufficiency in chronic pancreatits consists of the oral administration of a combination of pancreatic enzymes during meals. [8] Every patient with EPI and maldigestion, independent of the degree of steatorrhoea and presence or absence of associated symptoms, should receive pancreatic enzyme replacement therapy (PERT). The main focus in the management of EPI is to prevent weight loss, vitamin deficiencies, and to improve the nutritional status. The most important clinical parameter to monitor treatment efficacy is body weight. Several studies have demonstrated that in most patients one year of PERT resulted in a significant weight gain. However, abnormally low nutritional parameters have been demonstrated in approximately 70% of CP patients despite adequate clinical control with PERT
To avoid malnutrition related morbidity and mortality, it is pivotal to commence pancreatic enzyme replacement therapy as soon as EPI is diagnosed. Factors as early acidic inactivation of ingested enzymes, under dosage, and patient incompliance may prevent normalisation of nutrient absorption, in particular of fat digestion.
The typical indications for starting enzyme replacement therapy are progressive weight loss and steatorrhea, defined as at least 7 to 15 g of fecal fat per day, but there are no substantial data to support these guidelines. Since steatorrhea does not typically occur until >90% of pancreatic lipase activity is lost, 10% enzyme activity is the initial goal for therapy. Dosing is adjusted based on the amount of lipase in the supplements, and the initial dose aims at supplying 40 to 60 IU/minute of lipase activity within the duodenal lumen. To achieve this goal in adults, approximately 25,000 to 40,000 IU of lipase is required to digest a typical meal, and about 5000 to 25,000 IU of lipase per snack. [8]
In general, PERT is regarded as safe with few side effects, and adverse events are comparable to those with placebo. Supplemental enzymes act within the lumen of the intestine, and this is considered an intraluminal and not a systemic therapy. The most commonly reported side effects for recently approved enzymes are headache (6%), dizziness (6%), abdominal pain (9%), and flatulence.
Nutrition in chronic pancreatitis
According to the definition, the severity of malnutrition is correlated with two major factors: depletion of nutrients (alcoholism and pain) and malabsorption causes impaired nutritional status and increased metabolic activity due to the inflammatory component of CP (severity of disease). Generally patients at nutritional risk have an increased number of complications and a poorer outcome, but specific studies investigating this issue in CP are however not available.
A persistent alcohol intake, pain after a meal and maldigestion are the main causes of weight loss, and weight loss is strongly associated with maldigestion of fat.
Assessment of nutrition status should include a multidisciplinary approach, including assessment of clinical symptoms, exocrine and endocrine pancreatic function, body composition, bone health, dietary evaluation and lifestyle as illustrated in Figure 1. [7]
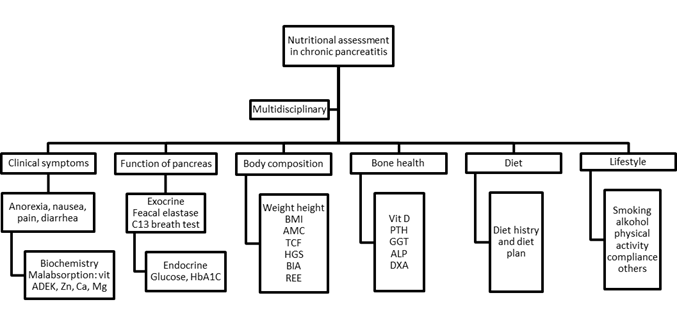
Figure 1: Diet recommendations
Micronutrient Deficiency
Micronutrient deficiencies may be present in chronic pancreatitis even in patients with reasonable nutrition. [5]
It can be caused by several mechanisms, like: suboptimal dietary intakes, increased losses of micronutrients, increased needs, antioxidant activity, and malabsorption. Vitamin E deficiency in CP may be up to 75% patients. Vitamin E deficiency may occur more often than deficiencies of vitamin A, D, and K.
According to guidelines on enteral nutrition in pancreatitis fat soluble vitamins (A,D,E,K) as well as other micronutrients should be supplemented if clinical deficit is apparent. Parenteral injection of lipophylic vitamins is strongly recommended in patients with severe exocrine pancreatic insufficiency. [19]
Vitamin B12 deficiency can occur in patients with CP due to inadequate protease secretion by the pancreas, which is required to release the vitamin for absorption in the terminal ileum.
Also it can occur clinically significant zinc deficiency, more common when associated with diabetes. [5]
Patients with chronic pancreatitis are at increased risk for osteoporosis. Osteopathy (osteoporosis, osteomalacia, osteopenia) may occur in 1 in 4 chronic pancreatitis patients, due to malabsorption of vitamin D and calcium, poor dietary intake, immobility, smoking, alcoholism and other factors. Vitamin D is essential for active intestinal calcium absorption, playing an essential role in maintaining calcium homeostasis and skeletal integrity. Lack of sunlight increases the need for dietary sources of vitamin D, which include fatty fish and liver as well as foods fortified with vitamin D, such as milk, juice, and cereals. Supplementation may be required if dietary and sunlight sources are inadequate. In most cases, deficiency or insufficiency of vitamin D is asymptomatic. In osteomalacia following prolonged deficiency, skeletal pain and muscle weakness may be present. Biochemically, serum levels of vitamin D, calcium, and phosphate may be low, and alkaline phosphatase may be elevated. Hypersecretion of parathyroid hormone (PTH), and therefore elevated PTH levels, may be one of the earliest signs of insufficient vitamin D stores. In CP, vitamin D deficiency appears to be related to the degree of exocrine insufficiency and therefore to the severity of disease. In a study „Patients with chronic pancreatitis are at increased risk for osteoporosis” sixty-two patients (mean age, 47.9 years; 72.6% male) and 66 matched controls were recruited. Dual-energy x-ray absorptiometry, smoking, and socioeconomic data were recorded. [18]
Thirty-four percent of patients had osteoporosis compared to 10.2% of controls. T-scores at the right femoral neck were lower in patients than controls (P = 0.005). Patients in the highest smoking tertile had the poorest T-scores at the lumbar vertebrae and total hip. Patients in the youngest age tertile had the highest T-scores (P = 0.003), but there was no sex difference.
Patient osteoporosis rates were triple that of controls, and almost 7 times what has been previously reported. Given the resource burden of osteoporosis, we suggest that routine bone density assessment is performed in patients with chronic pancreatitis.
Osteoporosis is a major public health problem because of its potentially severe consequences for both patients and the health care system. Because osteoporosis is a preventable disease, bone health guidelines for other gastrointestinal conditions recommend routine BMD assessment and supplementation to reduce the risk of osteopathy. [16]
Nutrition Requirements
Dietary recommendations begin with total abstinence from alcohol. In addition a high calorie intake of 35 kcal/kg/d is warranted. Resting energy expenditure in CP may be higher than normal by 30%-50%. It may be higher in underweight CP patients (35 ± 0.9 kcal/kg/d) compared with normal weight CP patients. [15] There is evidence that patients with chronic pancreatic exocrine insufficiency will salvage malabsorbed energy by colonic bacterial metabolism. For individuals with CP, 30% of total calories can be given as fat. The ESPEN guidelines state that vegetable fat may be better tolerated than animal fat in CP; however, this is unsupported by a reference from the literature. A severe restriction in dietary fat content to alleviate fat malabsorption or pain is commonly advised but is not always appropriate and may lead to malnutrition in the already nutritionally at-risk patient. Fat intake induces the release of cholecystokinin (CCK) in the duodenum, which stimulates secretions from the pancreatic exocrine gland Therefore, it is theoretically reasonable to restrict oral fat intake to prevent painful relapses. Indeed, a low-fat enteral supplement has been shown to minimally increase plasma CCK levels, leading to reduced postprandial pain in CP.[17]
To aid weight gain, particularly in the presence of steatorrhea, supplementation with a medium-chain triglyceride (MCT) fat source may be useful. MCT are absorbed directly across the small bowel into the portal vein, even in the absence of lipase, co-lipase and bile salts. However, MCTs have low energy density and unpalatable taste, and a maximum of about 50 g/d might be given. Higher doses may be ketogenic and are associated with side effects such as cramps, nausea and diarrhea. MCT intake should be increased slowly according to tolerance. [14,17]
A diet rich in carbohydrates and protein is generally advised, although carbohydrates may need to be limited in the case of concurrent diabetes. A protein intake of 1-1.5 g/kg is sufficient and well tolerated. A low-fiber diet is generally recommended, as fiber may absorb enzymes and thus delay the absorption of nutrients.
The best clinical follow-up parameters for monitoring therapeutic success of dietary counseling are improvement of the patient’s general condition and weight gain.
Nutrition after pancreatectomy
Preoperative nutrition status has been shown to influence surgical outcomes. This technically demanding operation involves an extensive surgical resection and alters digestive processes, which can influence nutrition long term. Searches of databases yielded 10 studies examining nutritional support in 571 patients undergoing pancreaticoduodenectomy. Data were retrieved on proportion of pre-operative weight loss, biochemical parameters (pre-operative albumin and presence of jaundice), type and duration of nutritional support, and clinical outcome (morbidity, mortality, and hospital stay). Pre-operative percentage weidge loss was similar in all studies evaluated. Routine post-operative total parenteral nutrition (TPN) was associated with a higher incidence of complications. Enteral nutrition reduced infective complications. Cyclical nutrition was associated with a lower incidence of post-operative gastric stasis. It was conluded that patients undergoing pancreaticoduodenectomy are nutritionally depleted at the time of surgery and the pre-operative period may present a window for intervetion. Despite that routine TPN is not beneficial. Routine post- operative enteral nutritional support, delivered on a cyclical basis appears to be the optimal mode of delivery.
Summary
Patients with chronic pancreatitis are at risk of malnutrition and nutrient deficiencies. Malnutrition is a huge problem in population, especially in hospitalised patients. Routine assessment and regular monitoring of nutrition status is essential.
Malnutrition, which is often overlooked by clinicians, is common and has wide-ranging effects on physiological function. It is associated with increased rates of morbidity and mortality in hospital patients and significantly increases healthcare costs. Implementation of a simple screening tool identifies patients at risk and allows appropriate treatment to be instituted; this can significantly improve clinical outcomes and reduce healthcare expenditure. Every doctor should know that proper nutritional care is essencial to good clinical practice.
World Health Organization Definition
Lean M, Wiseman M; Malnutrition in hospitals BMJ. 2008 Feb 9; 336(7639): 290 PMid:18258936 PMCid:PMC2234526
View Article PubMed/NCBIBarker LA, Gout BS, Crowe TC; Hospital Malnutrition: Prevalence, Identification and Impact on Patients and the Healthcare System Int J Environ Res Public Health. 2011 Feb;8(2):514-27
Detsky AS, Mclaughlin JR, Baker JP, Johnston N, Whittaker S, Mendleson RA, Jeejeebhoy KN; What is subjective global assessment of nutritional status? JPEN J Parenter Enteral Nutr. 1987;11:8–13 PMid:3820522
View Article PubMed/NCBIRasmussen HH, Irtun Ø, Olesen SS, Drewes AM, Holst M. Nutrition in chronic pancreatitis. World J Gastroenterol. 2013 Nov 14; 19(42): 7267–7275 PMid:24259957 PMCid:PMC3831208
View Article PubMed/NCBIFood and Agriculture Organisation of the United Nations; Consequences of Malnutrition and Hunger
View ArticleDuggan S, O'Sullivan M, Feehan S, Ridgway P, Conlon K; Nutrition treatment of deficiency and malnutrition in chronic pancreatitis: a review. Nutr Clin Pract. 2010 Aug; 25(4):362-70 PMid:20702842
View Article PubMed/NCBIde la Iglesia-García D, Huang W, Szatmary P, Baston-Rey I, Gonzalez-Lopez J, Prada-Ramallal G, Mukherjee R, Nunes QM, Domínguez-Mu-oz JE, Sutton R; NIHR Pancreas Biomedical Research Unit Patient Advisory Group1; Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut. 2017 Aug;66(8):1354-1355 PMid:27941156 PMCid:PMC5530474
View Article PubMed/NCBISikkens EC, Cahen DL, Kuipers EJ, Bruno MJ; Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010 Jun;24(3):337-47 PMid:20510833
View Article PubMed/NCBIFieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol. 2011; 4: 55–73 PMid:21753892 PMCid:PMC3132852
PubMed/NCBIPongprasobchai S; Maldigestion from pancreatic exocrine insufficiency. J Gastroenterol Hepatol. 2013 Dec; 28 Suppl 4:99-102 PMid:24251713
View Article PubMed/NCBIHackert T, Schütte K, Malfertheiner P; The Pancreas: Causes for Malabsorption. Viszeralmedizin 2014 Jun; 30(3): 190–197 PMid:26288593 PMCid:PMC4513827
View Article PubMed/NCBILi, Z.-S., Liao, Z., Chen, J.-M., Férec C ; Chronic Pancreatitis From Basic Research to Clinical Treatment. 2017, Springer Nature Singapore Pte Ltd. and Shanghai and Technical Publishers
Ikeura T, Takaoka M, Uchida, Miyoshi H, Okazaki K; Beneficial Effect of Low-Fat Elemental Diet Therapy on Pain in Chronic Pancreatitis. Int J Chronic Dis. 2014; 2014: 862091
Domínguez-Mu-oz JE, Phillips M. Nutritional Therapy in Chronic Pancreatitis. Gastroenterol Clin North Am. 2018 Mar;47(1):95-106 PMid:29413021
View Article PubMed/NCBIDuggan SN, O'Sullivan M, Hamilton S, Feehan SM, Ridgway PF, Conlon KC; Patients with chronic pancreatitis are at increased risk for osteoporosis. Pancreas. 2012 Oct;41(7):1119-24. PMid:22836855
View Article PubMed/NCBICasti-eira-Alvari-o M, Lindkvist B, Luaces-Regueira M, Iglesias-García J, Lari-o-Noia J, Nieto-García L, Domínguez-Mu-oz JE; The role of high fat diet in the development of complications of chronic pancreatitis. Clin Nutr. 2013 Oct;32(5):830-6 PMid:23453638
View Article PubMed/NCBIDuggan N, Smyth D, Murphy A, Macnaughton D, O'Keefe SJ, Conlon KC; High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014 Feb;12(2):219-28 PMid:23856359
View Article PubMed/NCBIBhardwaj P, Thareja S, Prakash S, Saraya A; Micronutrient antioxidant intake in patients with chronic pancreatitis. Gastroenterol. 2004 Apr-Jun;25(2):69-72.
Saunders J, Smith T; Malnutrition: causes and consequences. Clinical Medicine 2010, Vol 10, No 6: 624–7
View ArticleLohr M, Andren-Sandberg A; Pacreatitis - Diagnosis and Therapy. 2011, English, Uni-Med Verlag Ag
