Jian Sun
Tel./fax: +86 771 3240692
E-mail: jiansun@gxaas.net
Xiangrong You
Tel./fax: +86 771 3278647
E-mail: youxiangrong@gxaas.net
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 8
Page No: 912-923
Jian Sun
Tel./fax: +86 771 3240692
E-mail: jiansun@gxaas.net
Xiangrong You
Tel./fax: +86 771 3278647
E-mail: youxiangrong@gxaas.net
Ping Wei1,2. Xiangrong You1,2,*. Jian Sun1,2,*. Yayuan Zhang1. Guoming Liu1, .Mingjuan Li1. Kui Zhou1 . Ying Wang1
1 Agro-food Science and Technology Research Institute, Guangxi Academy of Agricultural Sciences, 174 East Daxue Road, 530007 Nanning, China
2 Guangxi Key Laboratory of Fruits and Vegetables Storage-processing Technology, 530007 Nanning , China
Lucas D Dias(lucasdanillodias@gmail.com)
Carolina A Curti(ccurti@unsa.edu.ar)
Ping Wei, Xiangrong You*, Jian Sun*, Ping Wei, Yayuan Zhang, Guoming Liu, Mingjuan Li, Kui Zhou, Ying Wang, Optimal process of supercritical carbon dioxide extracting Bama hempseed oil and its physicochemical property (2019) Journal of Food Science & Technology 4(8) pp:912-923
The objective of this work was to obtain optimal SC-CO2 extracting BHS oil process, response surface methodology (RSM) was applied to analyze effects of extraction pressure, extraction temperature, CO2 flow rate and extraction time on oil yield. Quality indices, fatty acid compositions and antioxidant activities were further compared between SC-CO2-extracted oil and Soxhlet-extracted oil. The results showed that the maximum recovery of BHS oil was 26.78 ± 2.75% (w/w) when SC-CO2 conditions were extraction pressure of 38.8 MPa, extraction temperature of 36.15 °C, CO2 flow rate of 7.51 L/min and extraction time of 3 h. SC-CO2-extracted BHS oil exhibited better transparency, smell and color as well as higher unsaturated fatty acid content than Soxhlet-extracted oil. Oleic acid, linoleic acid and linolenic acid contents were 12.75 ± 0.90%, 56.51 ± 1.74% and 19.18 ± 2.69%, respectively. Additionally, SC-CO2-extracted BHS oil showed higher iodine value, saponification value and tocopherol, lower acid value and peroxide values as well as stronger antioxidant activity than Soxhlet-extracted oil. In conclusion, RSM was successfully applied for SC-CO2 extraction optimization of BHS oil. SC-CO2 extraction was a better technique to produce higher value BHS oil than traditional Soxhlet extraction.
Keywords Bama hempseed oil . Supercritical carbon dioxide extraction . Response surface methodology . Optimal processing . Fatty acid compositions
Bama hempseed (BHS), named from its origin Bama county (one of five longevity towns in the world) in Guangxi province, is a famous local hemp cultivar and longevity food, which is a medicine food homology plant with a history of more than 3000 years in China(Callaway, 2004). Recent studies have found that edible hemps contain many valuable components, including fatty acids, cannabidiol, β-caryophyllene, myrcene, β-sitosterol, α/γ-tocopherol and methyl salicylate (Deferne et al. 1996; Leizer et al. 2000). Some researchers further investigated healthy aspects of hempseed oil. Hempseed have been reported that contained the highest amounts of unsaturated fat among edible vegetable oils, which is rich in omega-3 and omega-6 fatty acid. Linoleic acid and linolenic acid ratio is 3:1 (Kriese et al.2004; Matthäus et al. 2008). This ratio is considered to be the best proportion for normal metabolism of human body(Kostić et al. 2013). These two polyunsaturated fatty acids possess potential health benefits owing to their anti-inflammatory, antithrombotic, antiarrhythmic and hypolipidemic properties(Simopoulos, 2002). Hempseed oil also contains appreciable amounts of tocopherols with antioxidant activity(Leger, 2000). Previous studies have found that this oil (grow in Canada) contains more than 800 mg/kg tocopherol, mainly existing in the form of γ-tocopherol (85%). γ-Tocopherol exhibits radical-scavenging and anti-cancer activities, and its antioxidant capacity is higher than α-tocopherol and β-tocopherol(Lampi et al. 1997; Oomah et al. 2002). Besides, hempseed oil plays a role in treating constipation, secondary hard stool, chronic pharyngitis, neurodermatitis and high blood pressure in clinical medicine(Oomah et al.2002; Zhou et al.2018; Wolf, 1997; Callaway et al.2005). The potentially therapeutic properties make it very attractive to food, nutraceutical and pharmaceutical industries. Unfortunately, there are few reports on physicochemical property or gbioactive composition of special BHS oil.
Supercritical carbon dioxide (SC-CO2) as a medium for extracting oils has been applied in food industry in recent years. It is an alternative to conventional extraction methods (typically including screw press or organic solvent extraction) for edible vegetable oils. SC-CO2 extraction presents a number of advantages such as non-solvent residue and good retention of aromatic compounds. SC-CO2 can extract oil and protein simultaneously with minimal impact on environment, which is safer and more environment-friendly than solvent extraction(Maran et al. 2013). However, pure CO2 is not an appropriate extraction fluid for polar analytes and ethanol is needed to enhance the polarity of supercritical carbon dioxide. Previous studies have shown that SC-CO2 extraction gives a low amount of yield as compared with Soxhlet extraction(Putra et al. 2018) . What is more, SFE were fewer used for high quality hemp oil and other edible oils mainly due to very high investment costs of SFE equipment.(Aladic´ et al. 2015).
As for extraction approach of hempseed oil, the previous reports focus mostly on organic solvent extraction, ultrasonication, enzyme-assisted aqueous extraction and enzyme-assisted cold-pressing(Latif et al. 2009; Torres-Salas et al. 2014). Presently, various authors adopted SFE for hemp oil extraction, however, the process parameters of SFE were conside too simple (Da Porto et al. 2012; Aladic et al. 2015). Response surface methodology (RSM) is a collection of mathematical and statistical techniques that are useful for the modeling and analysis of problems in which a response of interest is affected by several variables and the objective is to optimize this response(Latif et al. 2009; Stroescu et al. 2013). Until now, information on RSM optimization of SC-CO2 extracting hempseed oil is limited. From previous studies, RSM had been used to optimize temperature, pressure and particle diameter on hemp seed (grow in Italy) oilextraction yield and oxidation stability. (Da Porto et al. 2012)and RSM was aslo conducted to study the effects of temperature, frequency, and nozzle size on hemp seed (grow in Croatia) oil recovery and quality parameters(Aladic et al. 2015). From the results, main parameters of SFE are not comprehensive consider enough. Compared with the former researther, Devi relatively comprehensive reported central composite design to optimize the parameters ( i.e. temperature, pressure, CO2 flow rate, particle size, and co-solvent flow rate of CO2 flow rate) of supercritical fluid extraction (Devi et al. 2018). However, Devi discussed too little relationships of parameters on yield and quality of oil.
SC-CO2 extracting method of BHS oil was developed in the present study. Through comprehensive analyzing the effects of extraction pressure, extraction temperature, CO2 flow rate and extraction time on BHS oil yield, the extracting process was optimized using a five-level and four-variable central composite rotatable design from RSM. Furthermore, quality indices, fatty acid compositions and antioxidant activities were also compared between SC-CO2 extracting oil and Soxhlet extracting oil. The research consequences provide a scientific foundation for exploring safe, healthy and environment-friendly technique to produce higher value BHS oil.
2.1. Materials and chemicals
BHS (Bama hempseed)was obtained from Bama county of Guangxi province, China . The BHS were dried in an oven at 40 °C until the moisture level was constant (10 % w/w)and the dried BHS were ground to a powdered form using a domestic mixer and passed through a 40-mesh sieve. Commercial liquefied carbon dioxide (99.99% purity) was supplied by Guangxi Oxygen Co. (Nanning, Guangxi, China).Fatty acid standards were purchased from Sigma Chemical Co. (St Louis, MO, USA).The chemicals used were of analytical reagent grade that include 1,1-diphenyl-2-picrylhydrazil (DPPH), petroleum ether, ethanol etc.were purchased from Shanghai Yuanye Biochemical Regent Co., Ltd.
2.2. Soxhlet extraction
Soxhlet extraction was performed as positive control. Three grams of BHS powder (80-mesh) were extracted for 3 h at 40 °C using 100 mL of petroleum ether in a SZF-06A Soxhlet apparatus (Zhejiang Top Instrument Co., Hangzhou, Zhejiang, China). After extraction, solvent was evaporated from the extracted oil, dried for 1 h at 50 °C in an oven, and then stored at –4 °C until further analysis. Total oil yield (%) was calculated as follows: Oil yield (%) = (mass of extracted BHS oil / mass of BHS powder) ´ 100.
2.3. Supercritical fluid extraction (SFE)
The efficiency of SC-CO2 extraction was mainly affected by parameters of extraction pressure, extraction temperature, CO2 flow rate and extraction time except for particle diameter of experimental material. Multiple variables may influence oil output, and RSM is an effective technique for optimizing SC-CO2 extraction process(Yolmeh et al. 2017). The schematic diagram of SFE system (100 mL sample capacity, Model SFE-500M1-2, American Thar Co., Applied Separations Inc., Allentown, PA, USA) was used to extracted BHS oil. A total of 35 g of BHS powder were placed into a 200 × 30 mm extraction vessel. After an initial air purge, liquefied CO2 was pumped into the vessel by a high-pressure pump to a given pressure. The temperature inside vessel was raised and maintained at the desired level by a heating jacket outside vessel. Extraction pressure and extraction temperature were controlled to the accuracy of ±0.5 MPa and ±0.5 °C. CO2 flow rate was regulated by adjusting the length of pumping stroke. After extraction, BHS oil was collected into a transparent bottle and weighed to determine yield. It was used as sample for analyzing quality indices, fatty acid compositions and antioxidant activities.
2.4. Optimal extraction conditions of BHS oil
Central composite design by RSM was used to study effects of four independent variables (extraction pressure, extraction temperature, CO2 flow rate and extraction time) at five levels on BHS oil yield. Ranges and center points for these four independent variables were based on the results of preliminary experiments (Table 1). Central composite design consisted of 24 factorial points and six replicates of central point (Table 2). The behavior of the system was explained by the second degree polynomial according to following equation:
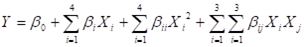
where Y was response function, β0 was intercept, and βi, βii and βij were coefficients of linear, quadratic and interactive terms, respectively. Xi and Xj were coded independent variables. The fitted polynomial equation was expressed as surface and contour plots to visualize relationship between responses and experimental levels of each factor. Design-Expert 8.05 (Stat-Ease Inc., Minneapolis, MN, USA) was used to determine the analysis of variance (ANOVA) and the coefficient of determination (R2) was used to estimate the fitness of model.
2.5. Quality attributes of the extracted BHS oil
Transparency, smell, color, oil gravity, refractive index, iodine value and saponification value of extracted BHS oil were determined using AOAC standard analytical methods(AOAC International, 2002). Iodine value was expressed as grams of iodine absorbed per 100 g of oil sample. Acid value and peroxide value were calculated through standard IUPAC methods(Paquot, 2013).
2.6. Fatty acids of the extracted BHS oil
Fatty acids in extracted BHS oil were analyzed on an Agilent 1100 gas chromatography (Agilent Technologies, Santa Clara, CA, USA) equipped with a fused silica capillary column (60 m × 0.25 mm × 0.25 μm, Trace Tr-fame, Thermo Fisher Scientific, Waltham, MA, USA) and a flame ionization detector (FID), according to the modified method of Yu et al(Yu. et al.2004) The oven temperature was initially set at 60 °C for 3 min, and then increased to 175 °C at a rate of 5 °C/min and kept for 15 min. Finally, it increased to 220 °C at a rate of 2 °C/min and kept for 10 min. A total of 1 µL of sample was injected with a split ratio of 1:100. Hydrogen and atmosophere were also injected at flow rates of 30 mL/min and 400 mL/min. Nitrogen was used as carrier gas at a flow rate of 25 mL/min. Fatty acid compositions were identified by comparing retention time with authentic compounds analyzed under the same conditions. Fatty acid contents were calculated from their peak areas.
2.7. Antioxidant activity of the extracted BHS oil
The radical scavenging activities of extracted BHS oil were measured according to the method of Han(Han et al. 2018). The reaction mixtures contained 2 mL of BHS oil in ethanol and 2 mL of 1,1-Diphenyl-2-picrylhydrazyl(DPPH) solution in ethanol(0.05 mM) in the test tube. The tubes were reacted for 30 min in darkness after vigorously shaking. The absorbance value of reactants was determined at 517 nm using a UV-visible spectrophotometer (GENESYS 10S UV-Vis, Thermo Fisher Scientific), which was denoted as A1, while the absorbance value of other mixtures, including the 2-mL sample solution and the 2 mL of ethanol, were denoted as A2. Then, we took 2 mL of ethanol and 2 mL of DPPH solution in a mixture and determined the absorbance value after the reaction, which was denoted as A0. At the same time, the control group of antioxidant Vitamin C was determined.DPPH free radical scavenging rate (%) was calculated as follows: DPPH free radical scavenging rate (%) = [1 − (A1 − A2) / A0] × 100.
2.8. Statistical analysis
All experimental results performed under CCD were analyzed using Design-Expert8.05 software (Stat-Ease, Inc., Minneapolis, MN, USA). Data were statistically analyzed via Duncan’s test using SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) and presented as means ± standard deviations obtained by triplicate experiments. A probability value of p < 0.05 was considered significant for differences among mean values.
Table 1. Independent variables and their levels used RSM design
|
|
|
|
|
Level |
|
|
|
Independent variables |
Symbol |
-2 |
-1 |
0 |
1 |
2 |
|
Extraction pressure (MPa) |
X1 |
25 |
30 |
35 |
40 |
45 |
|
Extraction temperature (°C) |
X2 |
30 |
35 |
40 |
45 |
50 |
|
CO2 flow rate (L/min) |
X3 |
5 |
6 |
7 |
8 |
9 |
|
Extraction time (h) |
X4 |
1.5 |
2 |
2.5 |
3 |
3.5 |
Table 2. Experimental design for five-level-four-factor central composite design and the obtained responses for SC-CO2 extracting BHS oil
|
Run |
X1 |
X2 |
X3 |
X4 |
Y (%) |
|
1 |
-1 |
-1 |
1 |
-1 |
9.05 ± 2.12 |
|
2 |
1 |
-1 |
-1 |
-1 |
12.01 ± 4.24 |
|
3 |
1 |
-1 |
1 |
-1 |
14.3 ± 0.71 |
|
4 |
0 |
0 |
2 |
0 |
15.78 ± 0.35 |
|
5 |
1 |
1 |
1 |
1 |
24.67 ± 0.91 |
|
6 |
-1 |
-1 |
1 |
1 |
18.28 ± 2.47 |
|
7 |
0 |
0 |
-2 |
0 |
9.46 ± 1.06 |
|
8 |
2 |
0 |
0 |
0 |
24.35 ± 0.34 |
|
9 |
0 |
0 |
0 |
0 |
21.3 ± 2.17 |
|
10 |
-2 |
0 |
0 |
0 |
14.89 ± 1.41 |
|
11 |
1 |
1 |
-1 |
-1 |
12.45 ± 1.53 |
|
12 |
-1 |
1 |
1 |
-1 |
12.56 ± 3.18 |
|
13 |
0 |
0 |
0 |
0 |
19.9 ± 3.77 |
|
14 |
1 |
-1 |
1 |
1 |
26.78 ± 2.75 |
|
15 |
0 |
0 |
0 |
-2 |
8.71 ± 0.35 |
|
16 |
-1 |
1 |
-1 |
-1 |
10.5 ± 1.41 |
|
17 |
-1 |
-1 |
-1 |
1 |
17.93 ± 2.83 |
|
18 |
1 |
1 |
-1 |
1 |
17.61 ± 1.77 |
|
19 |
1 |
1 |
1 |
-1 |
18.21 ± 2.47 |
|
20 |
0 |
0 |
0 |
0 |
19.4 ± 2.12 |
|
21 |
0 |
0 |
0 |
0 |
18.7 ± 0.32 |
|
22 |
-1 |
1 |
1 |
1 |
15.79 ± 1.50 |
|
23 |
0 |
2 |
0 |
0 |
15.48 ± 2.52 |
|
24 |
0 |
0 |
0 |
0 |
18.5 ± 1.08 |
|
25 |
1 |
-1 |
-1 |
1 |
22.38 ± 4.95 |
|
26 |
-1 |
1 |
-1 |
1 |
15.1 ± 0.80 |
|
27 |
0 |
0 |
0 |
0 |
19.2 ± 2.03 |
|
28 |
-1 |
-1 |
-1 |
-1 |
8.59 ± 0.20 |
|
29 |
0 |
-2 |
0 |
0 |
17.71 ± 3.22 |
|
30 |
0 |
0 |
0 |
2 |
24.37 ± 1.30 |
Y is the dependent variable.
3.1. Central composite design for SC-CO2-extracted BHS oil by RSM
The conditions for SC-CO2-extracted BHS oil were optimized by RSM using central composite design. Table 2 showed the design matrix and the obtained responses for extraction yield. BHS oil yield (Y) was calculated as follows: Y = 19.50 + 2.48X1 − 0.29X2 + 1.49X3 + 3.84X4 − 0.025X12 − 0.78X22 − 1.78X32 − 0.80X42 − 0.16X1X2 + X1X3 + 0.50X1X4 + 0.50X2X3 − 1.37X2X4 + 0.12X3X4, where Y was response variables, X1, X2, X3 and X4 were coded values of four independent variables (extraction pressure, extraction temperature, CO2 flow rate and extraction time), 19.5 was a constant, and other numbers were linear, quadratic and interactive coefficients. Three-dimensional surface response plots were generated by varying two variables within experimental range and holding the other two constant at central point. The coefficients of response surface equation were estimated by using Stat Graphics Centurion XV (Thermo Fisher Scientific). The test of statistical significance was based on total error criteria with a confidence level of 95%. BHS oil yields obtained from all experiments were also listed in Table 2. The experimental data were used to calculate coefficients of second-order polynomial equation. The obtained regression coefficients were summarized in Table 3. ANOVA showed that the resultant second-order polynomial model adequately represented experimental data with R2 equal to 0.9865. ANOVA also evaluated the significance of coefficients in model (Table 3). In this model, a large regression coefficient and a small P-value indicated obvious effects on respective response variables. Thus, the partial regression coefficient of independent variables (X1, X3 and X4), three quadratic terms (X22, X32 and X42), and the interaction terms of X2X4 were extremely significant (P < 0.01), suggesting that they had obvious effects on Y value. The partial regression coefficient of interaction terms (X1X3, X1X4, and X2X3) were significant (P < 0.05), suggesting that they also had obvious effects on Y value.
Table 3. ANOVA for recovery of SC-CO2 extracting BHS oil
|
Variable |
Sum of squares |
df |
Mean square |
F-value |
P-value |
|
Model |
715.90 |
14 |
51.14 |
78.15 |
< 0.0001** |
|
X1 |
147.66 |
1 |
147.66 |
225.67 |
< 0.0001** |
|
X2 |
1.98 |
1 |
1.98 |
3.02 |
0.1026 |
|
X3 |
53.13 |
1 |
53.13 |
81.21 |
< 0.0001** |
|
X4 |
354.12 |
1 |
354.12 |
541.22 |
< 0.0001** |
|
X1 X2 |
0.43 |
1 |
0.43 |
0.66 |
0.4290 |
|
X1 X3 |
15.90 |
1 |
15.90 |
24.30 |
0.0002** |
|
X1 X4 |
4.07 |
1 |
4.07 |
6.22 |
0.0248** |
|
X2 X3 |
4.07 |
1 |
4.07 |
6.22 |
0.0248** |
|
X2 X4 |
30.17 |
1 |
30.17 |
46.11 |
< 0.0001** |
|
X3 X4 |
0.23 |
1 |
0.23 |
0.36 |
0.5597 |
|
X12 |
0.017 |
1 |
0.017 |
0.026 |
0.8731 |
|
X22 |
16.75 |
1 |
16.75 |
25.59 |
0.0001** |
|
X32 |
86.43 |
1 |
86.43 |
132.09 |
< 0.0001** |
|
X42 |
17.34 |
1 |
17.34 |
26.50 |
0.0001** |
|
Residual |
9.81 |
15 |
0.65 |
|
|
|
Lack of fit |
4.67 |
10 |
0.47 |
0.45 |
0.8647 |
|
Pure error |
5.14 |
5 |
1.03 |
|
|
|
Correlation total |
725.72 |
29 |
|
|
|
** Significant at 95% confidence level, where f is a random variable generated from the statistic. df, degrees of freedom.
Response surfaces generated by proposed models expressed interactions between two independent variables. Figure 1a showed 3-D response plot of BHS oil yield under varying extraction pressure and extraction temperature at fixed extraction time (3 h) and CO2 flow rate (7.51 L/min). This yield significantly increased under extraction pressure of 25−40 MPa, the reason was that at high pressures the solubility of the oil increased due to the increase in density of CO2, leading to greater oil solubility in CO2.( Brunner, 1994; Molero Gomez et al. 2002; Da Porto et al. 2012). However, the oil yield gradually decreased under 40−45 MPa probably due to the fact that the highly compressed CO2 facilitates solute-solvent repulsion (Liu et al. 2009; Da Porto et al. 2012). Besides, owing to a positive correlation of hemp melanin dissolution with supercritical fluid extraction pressure, the higher pressure was, the earlier green grease with stimulation smell presented (Tomita et al. 2013). For this reason, high pressure is not always recommended (Yamini et al. 2008).
In addition, BHS oil yield increased at extraction temperature of 30−36.15 °C, and then indistinctively decreased at 36.15−50 °C, which was probably caused by oil viscosity changes at different temperatures. Oil viscosity decreased with extraction temperature rising, and thus the withdrawal of oil from BHS became easier at high temperature. High temperature accelerated CO2 flow rate so as to increase circulation speed. However, high temperature reduced distribution coefficient of BHS oil in supercritical CO2. Although CO2 circulation speed accelerated, total extraction yield of BHS oil decreased(Reverchon et al. 2006). Therefore, the interaction between extraction pressure and extraction temperature had no significant influence on BHS oil yield. Figure 1b showed 3-D response surface plot of BHS oil yield under varying extraction pressure and CO2 flow rate at fixed extraction temperature (36.15 °C) and extraction time (3 h). This yield rapidly increased at CO2 flow rate of 5−7.51 L/min, but gradually decreased beyond 7.51 L/min. Additionally, BHS oil yield rapidly increased under extraction pressure of 25−45 MPa, but reached a plateau beyond 38.8 MPa. Pressure curve indicated that at low CO2 flow rate, BHS oil yield increased when pressure rose. However, at high pressure, this yield declined with CO2 flow rate increasing, probably because the short contact time between BHS particles and CO2 reduced oil solubility. Figure 1c showed 3-D response surface plot of BHS oil yield under varying extraction time and extraction pressure at fixed extraction temperature (36.15 °C) and CO2 flow rate (7.51 L/min). This yield appeared initially rising and then decreasing trend with the rise of extraction pressure. It reached the maximum when extraction time and extraction pressure were 3 h and 38.8 MPa. The reason for oil yield decreasing at high pressure was that CO2 became very difficult to infiltrate into BHS and interaction time between solute and solvent was short. Thus, the interaction of extraction time and extraction pressure significantly affected BHS oil yield within a certain range. Figure 1d showed 3-D response surface plot of BHS oil yield under varying extraction temperature and CO2 flow rate at fixed extraction pressure (38.8 MPa) and extraction time (3 h). This yield reached the maximum when extraction temperature and CO2 flow rate were 36.15 °C and 7.51 L/min. The interaction of extraction temperature and CO2 flow rate significantly affected BHS oil yield. Figure 1e showed 3-D response surface plot of BHS oil yield under varying extraction temperature and extraction time at fixed extraction pressure (38.8 MPa) and CO2 flow rate (7.51 L/min). This yield reached the maximum within longer extraction time at low temperature, but contrarily reached the maximum within shorter extraction time at high temperature. BHS oil yield depended mainly on the competition between solute vapor pressure and supercritical fluid density. When extraction temperature rose, solute vapor pressure increased, and oil dissolving ability enhanced. However, the increasing temperature reduced supercritical fluid density, resulting in a decline of dissolve capacity of supercritical fluid. Thus, the interaction of extraction temperature and extraction time significantly affected BHS oil yield. Figure 1f showed 3-D response surface plot of BHS oil yield under varying CO2 flow rate and extraction time at fixed extraction temperature (36.15 °C) and extraction pressure (38.8 MPa). BHS oil yield significantly increased with CO2 flow rate and extraction time increasing, but the interaction between CO2 flow rate and extraction time on this yield was not significant. Because contact time between BHS and CO2 became shorter when flow rate increased, fat dissolution rate was lower, resulting in unobvious increase of oil yield.
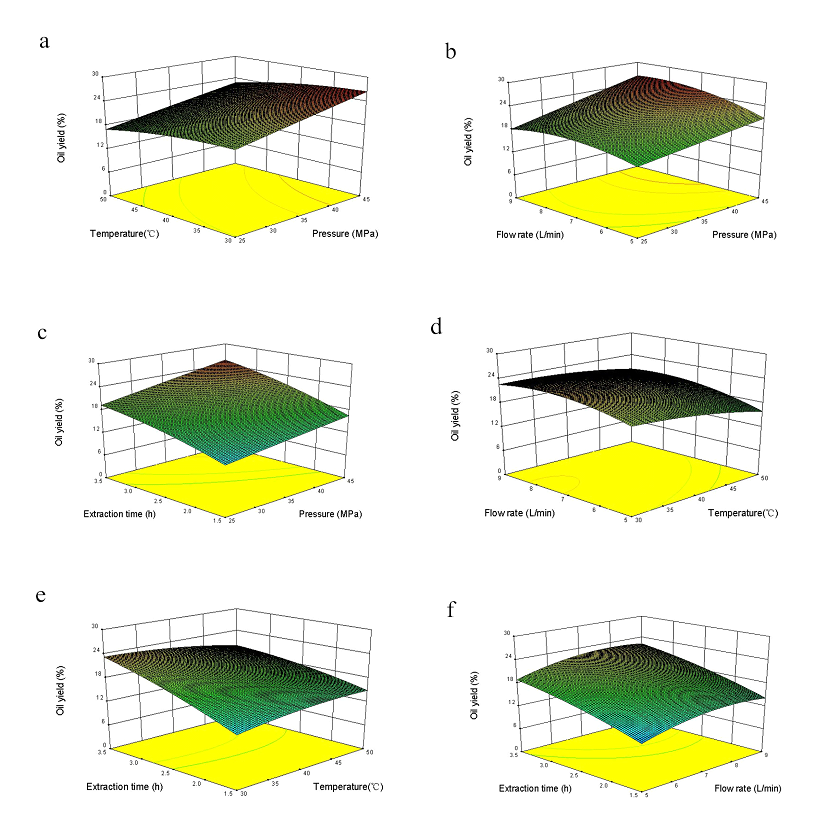
Fig.1 Response surface analysis of interaction items on recovery of SC-CO2 extracting BHS oil (shaking rate = 100 rpm). (a) interaction items of extraction temperature and extraction pressure; (b) interaction items of CO2 flow rate and extraction pressure; (c) interaction items of extraction time and extraction pressure; (d) interaction items of CO2 flow rate and extraction temperature; (e) interaction items of extraction time and extraction temperature; (f) interaction items of extraction time and CO2 flow rate.
3.2. Regression model and significance test for SC-CO2-extracted BHS oil
The variance analysis for SC-CO2-extracted BHS oil was performed to test the significance of regression model. The results showed that the significance of each coefficient was ascertained using F-test and associated P-value (Table 3). P-value could indicate interaction pattern between variables. The ANOVA of quadratic regression model demonstrated that the model was highly significant, because F-test exhibited a very low probability value (P < 0.0001). F-value of 78.15 implied that the model was significant. The ‘Lack-of-Fit F-value’ of 0.45 (> 0.05) implied that the ‘Lack-of-Fit’ was not significant. Furthermore, from Table 3, linear coefficients (X1, X3 and X4), quadratic term coefficients (X22, X32 and X42) and cross-product coefficients (X1X3, X1X4, X2X3 and X2X4) were all significant with very small P-values (P < 0.05). The other term coefficients were not significant (P > 0.05). Therefore, X1, X3, X4, X22, X32, X42, X1X3, X1X4, X2X3 and X2X4 represented the most important factors during BHS extraction process. In order to validate the credibility of quadratic regression model, R-Squared, Adj R-Squared, variation coefficient and other characteristic parameters were determined from Table 4. The coefficient of determination (R2) for data fit was 0.9865, the Adj R-Squared was 0.9739, and the coefficient of variation was 4.82%, respectively. These values indicated that polynomial model presented adequate accuracy and general availability. However, the reproducibility of model should be verified combining with actual production conditions.
Table 4. Credibility analysis of regression model
|
Item |
Std. Dev. |
Mean |
C.V (%) |
R2 |
Adj R2 |
Pred R2 |
Press |
Adeq Precision |
|
Numerical value |
0.81 |
16.80 |
4.82 |
0.9865 |
0.9739 |
0.9527 |
34.33 |
31.886 |
3.3. Optimal conditions for SC-CO2-extracted BHS oil
Design Expert 8.0.5 software was used to obtain regression equation in the scope of selected factors. X1, X2, X3 and X4 were used to calculate partial derivatives to determine the maximum yield and the optimal extraction conditions of BHS oil. In the present experimental ranges, the optimal extraction condition was predicted as extraction pressure of 38.8 MPa, extraction temperature of 36.15 °C, CO2 flow rate of 7.51 L/min and extraction time of 3 h. Under this condition, BHS oil yield was predicted to be 26.82%. Considering operational convenience, the actual condition for SC-CO2-extracted BHS oil was determined as extraction pressure of 40 MPa, extraction temperature of 35 °C, CO2 flow rate of 7.5 L/min and extracting time of 3 h. In order to verify this predicted result, SC-CO2-extracted BHS oil under above actual condition was repeated three times. The oil yield was calculated as 27.5% averagely, higher than oil yield of 22.1± 0.7% reported by Porto (Porto et al. 2012).which is basically accorded with the predictive value of 26.82 ± 2.54%, indicating that the predictive value of oil yield was fit with actual situation, and the model was suitable for optimizing processing parameters of SC-CO2-extracted BHS oil.
3.4. Quality indices of BHS oil
From Table 5, no significant differences were found in oil gravity, refractive index, acid value, saponification value, tocopherol and oil yield between SC-CO2-extracted BHS oil under optimal condition and Soxhlet-extracted BHS oil (positive control). However, comparing with positive control, SC-CO2-extracted oil presented higher transparent, lighter color and better smell (Fig. 2) as well as higher iodine value (Table 5). The reason attrubute to that SC-CO2 unlike organic solventis, which usually non-selective and causes the simultaneous removal of non-volatile pigments and waxes contaminated with solvent residues.( Da Porto et al. 2012). On the contrary, SC-CO2-extracted oil showed obviously lower peroxide value than positive control, indicating that SC-CO2-extracted oil was not easier to be oxidized than Soxhlet-extracted oil. In sum, SC-CO2-extracted BHS oil exhibited high transparency, good color, pleasant smell and low peroxide value, indicating that it had better sensory attributes and physicochemical properties than traditional Soxhlet-extracted oil. This result was similar to hemp seed oil (Da Porto et al. 2012) and other differrent vegetable oils (Bernardo-Gil et al.2004). Considering environmental and health-related problems of petroleum ether as Soxhlet extraction solvent, BHS oil for its potential health benefits is a special oil and its corresponding higher value make its extraction using SC-CO2 an economically viable option(Da Porto et al. 2012).

Fig.2 BHS oil extracted by different methods. (a) BHS oil extracted by SC-CO2; (b) BHS oil extracted by petroleum ether.
Table 5. Quality indices of BHS oil under different extraction processes
|
Parameter |
Soxhlet-extracted oil |
SC-CO2-extracted oil |
|
Transparency |
Little turbidity |
Transparent |
|
Smell |
Hemp oil fragrance with unpleasant smell |
Hemp oil fragrance |
|
Color (Color slot: 25.4 mm) |
Y58.2R6.3B5.0 |
Y42.3R2.1B1.2 |
|
Oil gravity (20 °C) |
0.935 ± 0.002 |
0.933 ± 0.002 |
|
Refractive index (n20) |
1.477 |
1.478 |
|
Acid value (mg/g) |
1.78 ± 0.07 |
1.74 ± 0.23 |
|
Iodine value (g/100 g) |
144.42 ± 0.37 |
164 ± 0.16 |
|
Saponification value (mg/g) |
186 ± 0.18 |
188 ± 0.35 |
|
Peroxide value (mmol/kg) |
16.48 ± 0.15 |
4.8 ± 0.18 |
|
Tocopherols (mg/100 g) |
37.2 ± 0.32 |
38.3 ± 0.12 |
|
Oil yield (%, w/w) |
30.06 ± 0.26 |
26.18 ± 0.5 |
3.5. Fatty acid compositions of BHS oil
Fatty acid profiles were compared between SC-CO2-extracted BHS oil under optimal condition and Soxhlet-extracted BHS oil (positive control). No significant difference in fatty acid compositions was found between SC-CO2-extracted oil and positive control.Others authors obtained similar results from hemp seed oil(Da Porto et al. 2012)and other differrent vegetable oils(Devittori et al.2000; Bernardo-Gil et al., 2004). Six main fatty acid components, i.e. three saturated fatty acids, one monounsaturated fatty acids and two polyunsaturated fatty acids, were identified from BHS oil using gas chromatography (Table 6). Polyunsaturated fatty acids (accounting for 75.69 ± 4.08%) dominated fatty acids in this oil, followed by monounsaturated fatty acids (accounting for 12.75 ± 0.90%), while saturated fatty acids content (accounting for 9.43 ± 0.08%) was the lowest.
All BHS oil samples exhibited high amounts of total unsaturated fatty acid in the present study. Its content in SC-CO2-extracted oil (88.44 ± 4.98%) was higher than that in positive control (85.12 ± 8.96%). Hemp seed oil usually contains abundant polyunsaturated fatty acids such as linolenic acid and linoleic acid(Deferne, 1996).The main polyunsaturated fatty acids in BHS oil was linoleic acid (56.51 ± 1.74% for SC-CO2-extracted oil and 56.19 ± 4.69% for positive control), which was significantly higher than linolenic acid (19.18 ± 2.69% for SC-CO2-extracted oil and 19.52 ± 4.01% for positive control). In addition, linoleic acid in BHS oil was also higher than that in common Chinese hemp varieties such as Gansu variety (about 49.77%) and Ningxia variety (about 51.34%)(Yu et al. 2009). The main monounsaturated fatty acids in BHS oil was oleic acid. The concentration of oleic acid in SC-CO2-extracted oil (12.75 ± 0.93%) was significantly higher than that in positive control (9.41 ± 0.31%). The main saturated fatty acids in BHS oil was palmitic acid (6.28 ± 0.08% for SC-CO2-extracted oil and 11.41 ± 0.18% for positive control). Two other saturated fatty acids, i.e. stearic acid (2.75 ± 0.15% for SC-CO2-extracted oil and 2.34 ± 0.33% for positive control) and arachidic acid (0.40 ± 0.15% for SC-CO2-extracted oil and 0.38±0.07% for positive control), were also identified from BHS oil. From above results, fatty acid compositions in SC-CO2-extracted BHS oil were similar to Soxhlet-extracted BHS oil. Comparing with Soxhlet-extracted oil, SC-CO2-extracted oil contained higher contents of unsaturated fatty acids, which made it superior for polymerization and modification due to more unsaturated double bonds in fatty acid chain(Da Porto et al.2012).
Table 6. Fatty acid compositions of BHS oil under different extraction processes
|
Fatty acids |
Soxhlet-extracted oil |
SC-CO2-extracted oil |
|
C16:0 |
11.41 ± 0.18 |
6.28 ± 0.08 |
|
C18:0 |
2.34 ± 0.33 |
2.75 ± 0.15 |
|
C18:1 |
9.41 ± 0.31 |
12.75 ± 0.93 |
|
C18:2 |
56.19 ± 4.69 |
56.51 ± 1.74 |
|
C18:3 |
19.52 ± 4.01 |
19.18 ± 2.69 |
|
C20:0 |
0.38 ± 0.07 |
0.40 ± 0.15 |
|
Monounsaturated fatty acid |
9.41 ± 0.31 |
12.75 ± 0.90 |
|
Polyunsaturated fatty acid |
75.71 ± 8.66 |
75.69 ± 4.08 |
|
Unsaturated fatty acid |
85.12 ± 8.96 |
88.44 ± 4.98 |
|
Saturated fatty acid |
14.13 ± 0.24 |
9.43 ± 0.08 |
C16:0, palmitic acid; C18:0, stearic acid; C18:1, oleic acid; C18:2, lenoleic acid; C18:3, linolenic acid; C20:0, arachidic acid. All data were expressed as mean ± standard deviation.
3.6. Antioxidant activities of BHS oil
The differences of antioxidant activities between SC-CO2-extracted BHS oil under optimal condition and Soxhlet-extracted BHS oil (positive control) were determined using DPPH radical scavenging capacity method. From Fig. 3, SC-CO2-extracted oil showed higher DPPH radical scavenging rate (66.62 ± 0.80%) than positive control (62.31 ± 0.56%), which indicated it possessed better antioxidant activity. Similar results that hemp seed oil extracted by supercritical CO2 exhibits the highest value of RSC (46.7 ± 3.1 µM) corresponding to 1.87 α-tocopherol equivalents/ml oil, about two-fold higher thanvergin olive oil (20.4 ± 5.6) had been reported by Da Porto(Da Porto et al. 2012). Since SC-CO2 extraction was performed at low temperature, which reduced thermal degradation of antioxidant compounds in BHS oil. As some researchers reported, the antioxidant potential of plant oils was mainly attributed to polyunsaturated fatty acids, vitamin E homologues (e.g. tocopherols)(Ramadan et al.2006; Putra et al. 2018). In vegetable oils, tocopherols are the most important natural antioxidants present (Kamal-Eldin et al.1996). In this study, BHS oil possessed relatively high content of polyunsaturated fatty acids (75.69±4.08%)and tocopherols(38.3±0.12 mg/100g). Though, the content of polyunsaturated fatty acids (75.69±4.08%) and tocopherols (38.3±0.12 mg/100g) have found lower than polyunsaturated fatty acids(81.08%, grow in Italy)) and tocopherol (800 mg/kg)of hemp seed oil (grow in Canada) reported by Da Porto and Oomah et al(Da Porto et al.2012; Oomah et al.2002). However, polyunsaturated fatty acids and tocopherols are still probably contributed to the antioxidant activity of BHS oil.
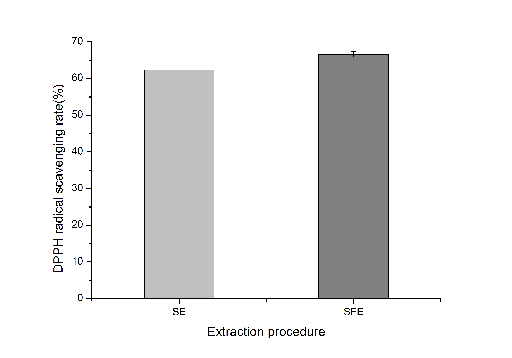
Fig.3 DPPH radical scavenging activities of BHS oil under different extraction processes. SE, Soxhlet-extracted oil; SFE, SC-CO2-extracted oil. Data were expressed as mean ± standard deviation.
Second-order poly-nomial model was sufficient to describe and predict the responses of BHS oil yields with SC-CO2 extraction process varies in the present experimental ranges. Extraction pressure, CO2 flow rate and extraction time independently and significantly affected BHS oil yield. In addition, the interaction between extraction temperature and extraction time had a significant influence on the oil yield. The graphical optimization result predicted that the optimal extraction condition in the present experimental ranges was extraction pressure of 38.8 MPa, extraction temperature of 36.15 °C, CO2 flow rate of 7.51 L/min and extraction time of 3 h. SC-CO2-extracted oil yield under this condition was similar to Soxhlet-extracted oil. Furthermore, SC-CO2-extracted oil exhibited higher transparency, better color, more pleasant smell, higher iodine value, lower peroxide value, more unsaturated fatty acid and stronger antioxidant activity than Soxhlet-extracted oil, indicating that SC-CO2 extraction was a better technique to produce healthy, environment-friendly and higher value BHS oil than traditional Soxhlet extraction.
The authors would like to acknowledge support by Science and Technology Major Project of Guangxi (Grant Nos. Gui Ke AA17204042, Gui Ke AA17204038, Gui Ke AA17202029), Bagui Scholars Project Special Fund (Grant No. [2016]21), Ministry of Personnel of China (Grant No. Ren She Ting Han [2015]192), Guangxi Natural Science Foundation (Grant No.2016GXNSFBA380167), Foundation of Fundamental Research Project from Guangxi Academy of Agricultural Sciences (2017JM52) and Dominant Discipline Team Project (Grant No. Gui Nong Ke 2015YT87, 2018YT29, 2018YM05).
Aladić K, Jarni K, Barbir T, Vidović S, Vladić J, Bilić M, Jokić S (2015) Supercritical CO2 extraction of hemp (Cannabis sativa L.) seed oil. Ind Crop Prod 76:472-478.
View ArticleAOAC International (2002) Official Methods of Analysis of AOAC International. Association of Official Analytical Chemist, Washington DC, USA.
Brunner G (1994) Gas extraction: an introduction to fundamentals of supercritical fluids and the application to separation processes, in: H. Baumgurtel, E.U. Franck(Eds.). Topic in Physical Chemistry, Springer, New York.
Bernardo-Gil MG, Lopes LMC (2004) Supercritical fluid extraction of Cucurbita fificifolia seed oil. Eur Food Res Technol 219(6):593-597.
View ArticleCallaway JC (2004) Hempseed as a nutritional resource: An overview. Euphytica 140(1-2):65-72 .
View ArticleCallaway J, Schwab U, Harvima I, Halonen P, Mykkänen O, Hyvönen P ,Järvinen T (2005) Efficacy of dietary hempseed oil in patients with atopic dermatitis. J Dermatolog Treat 16(2):87-94.
View ArticleDeferne JL, Pate DW (1996) Hemp seed oil: A source of valuable essential fatty acids. J Int Hemp Assoc 3(1):4-7.
View ArticleDa Porto C, Decorti D, Tubaro F (2012) Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind Crop Prod 36:401-404.
View ArticleDa Porto C, Voinovich D, Decorti D, Natolino A (2012) Response surface optimization of hemp seed (Cannabis sativa L.) oil yield and oxidation stability by supercritical carbon dioxide extraction. J Supercrit Fluid 68:45-51.
View ArticleDevi V, Khanam S (2018) Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J Clean Prod.
View ArticleDevittori C, Gumy D, Kusy A, Colarow L, Bertoli C, Lambelet P (2000) Supercritical fluid extraction of oil from millet bran. J Am Oil Chem Soc 77(6):573-579.
View ArticleHan CP, Liu QG, Jing Q, Wang D, Zhao Y, Zhang H, Jiang LZ (2018) Ultrasound-assisted aqueous enzymatic extraction of corn germ oil: analysis of quality and antioxidant activity. J Oleo Sci 67(6) :745-754.
View ArticleKriese U, Schumann E, Weber WE, Beyer M, Brühl L, Matthäus B (2004) Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. genotypes. Euphytica 137(3):339-351.
View ArticleKostić MD, Joković NM, Stamenković OS, Rajković KM, Milić PS, Veljković VB (2013) Optimization of hempseed oil extraction by n-hexane. Ind Crop Prod 48: 133-143.
View ArticleKamal-Eldin A, Appelqvist LA (1996) The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 31 (7):671-701.
View ArticleLeizer C, Ribnicky D, Poulev A, Dushenkov S , Raskin I (2000) The composition of hemp seed oil and its potential as an important source of nutrition. J Nutraceuticals Funct Med Foods 2(4):35-53.
View ArticleLeger CL (2000) Vitamin E:current state of knowledge, role in the prevention of cardiovascular disease, bioavailability. Ol Corps Gras Li 7(3) :258-265.
View ArticleLampi AM, Hopia A , Piironen V (1997) Antioxidant activity of minor amounts of γ-tocopherol in natural triacylglycerols. J Am Oil ChemSoc 74(5):549-555.
View ArticleLatif S, Anwar F (2009) Physicochemical studies of hemp (Cannabis sativa) seed oil using enzyme-assisted cold-pressing. Eur J Lipid Sci Technol 111(10):1042-1048.
View ArticleLiu SC, Yang F, Zhang CH, Ji HW, Hong PZ, Deng CJ (2009) Optimization of process parameters for supercritical carbon dioxide extraction of Passiflflora seed oil by response surface methodology. J Supercrit Fluid 48(1):9-14.
View ArticleMatthäus B, Brühl L (2008) Virgin hemp seed oil: An interesting niche product. Eur J Lipid Sci Technol 110(7):655-661.
View ArticleMolero Gómez A, Martínez de la Ossa E (2002) Quality of borage seed oil extractedby liquid and supercritical carbon dioxide. Chem Eng J 88(1-3):103-109. 00260-1
View ArticleOomah BD, Busson M, Godfrey DV , Drover JCG (2002) Characteristics of hemp (Cannabis sativa L.) seed oil. Food Chem 76(1):33-43.
View ArticlePrakash Maran J, Manikandan S, Priya B, Gurumoorthi P (2015) Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea(Camellia sinensis L.) leaves. J Food Sci Tech 52(1):92-104.
View ArticlePutra NR, Rizkiyah DN, Zaini AS, Yunus MAC, Machmudah S, Idham Z, Hazwan Ruslan MS (2018) Effect of particle size on yield extract and antioxidant activity of peanut skin using modified supercritical carbon dioxide and soxhlet extraction. J Food Process Preserv 42(8):e13689.
View ArticlePaquot C (2013) Standard methods for the analysis of oils, fats and derivatives, IUPAC commission on oils, fats and derivatives. Pergamon, London.
Reverchon E, De Marco I (2006) Supercritical fluid extraction and fractionation of natural matter. J Supercrit Fluid 38(2):146-166. https:// doi.org/10.1016/j.supflu.2006.03.020
Ramadan MF, Moersel JT (2006) Screening of the antiradical action of vegetable oils. J Food Comp Anal 19(8):838-842.
View ArticleSimopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365-379.
View ArticleStroescu M, Stoica-Guzun A, Ghergu S, Chira N, Jipa I (2013) Optimization of fatty acids extraction from Portulaca oleracea seed using response surface methodology. Ind Crop Prod 43:405-411.
View ArticleTorres-Salas P, Pedrali A, Bavaro T, Ambrosini S, Marrubini G, Pappalardo VM, Massolini G, Terreni M , Ubiali D (2014) Preparation of PUFA concentrates as acylglycerols via enzymatic hydrolysis of hempseed oil (Cannabis sativa L.) in a homogeneous low-water medium. Eur J Lipid Sci Technol 116(11):1496-1504.
View ArticleTomita K, Machmudah S, Quitain AT, Sasaki M, Fukuzato R, Goto M (2013) Extraction and solubility evaluation of functional seed oil in supercritical carbon dioxide.J Supercrit Fluid 79:109-113.
View ArticleWolf G (1997) σ-Tocopherol: an efficient protector of lipids against nitric oxide-initiated peroxidative damage. Nutr Rev 55(10):376-378.
View ArticleYolmeh M ,Jafari SM (2017) Applications of response surface methodology in the food industry processes. Food Bioprocess Technol 10(3):1935-5130.
View ArticleYu W, Zhao Y, Chen J , Shu B (2004) Comparison of two kinds of pumpkin seed oils obtained by supercritical CO2 extraction. Eur J Lipid Sci Tech 106:355-358.
View ArticleYamini Y, Khajeh M, Ghasemi E, Mirza M , Javidnia K (2008) Comparison of essentialoil compositions of Salvia mirzayanii obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem 108:341-346.
View ArticleYu JQ, Yu XZ, Chen XY , Du SK (2012) Physicochemical properties of hemp seed and its oils. China Oil Fat 37(4):84-87. (In Chinese)
Zhou YF, Wang SS, Lou HX,Fan PH (2018) Chemical constituents of hemp (Cannabis sativa L.) seed with potential anti-neuroinflammatory activity. Phytochem Lett 23:57-61. https:// dx.doi.org/10.1016/j.phytol.2017.11.013
