C. I. Kothe,
MICALIS Institute, INRA, Domaine de Vilvert, 78350 -Jouy-en-Josas, France.
E-mail: Caroline.Kothe@inrae.fr
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 5 ISSUE: 5
Page No: 244-251
C. I. Kothe,
MICALIS Institute, INRA, Domaine de Vilvert, 78350 -Jouy-en-Josas, France.
E-mail: Caroline.Kothe@inrae.fr
Regina Zilio1, Caroline Isabel Kothe1*, Tatiana Pacheco Soares Zamboni2, Cesar Aguzzoli2, Leticia Sopeña Casarin3, Eduardo César Tondo1
1 Departamento de Ciências dos Alimentos, Instituto de Ciência e Tecnologia de Alimentos, Universidade Federal do Rio Grande do Sul (ICTA/UFRGS), Av. Bento Gonçalves, 9500, 91501-970 Porto Alegre, RS, Brazil.
2 Área do Conhecimento de Ciências Exatas e Engenharias, Universidade de Caxias do Sul, Rua Francisco Getúlio Vargas, 1130, 95070-560, Caxias do Sul, RS, Brazil.
3 Departamento de Nutrição, Universidade Federal de Ciências da Saúde de Porto Alegre, Rua Sarmento Leite, 245, 90050-170, Porto Alegre, RS, Brazil.
Zilio R, Kothe CI, Zamboni TPS, Aguzzoli C, Casarin LS,Tondo EC, (2020) Silver implantation on AISI 304 stainless steel surface using low-energy doses and the antimicrobial effect against Salmonella Enteritidis and Listeria monocytogenes. Journal of Food Science & Technology 5(6) pp: 244-251
Silver has antimicrobial properties and when implemented on the stainless steel surface can inactivate microorganisms and consequently prevent biofilm formation and cross-contamination of food. Therefore, in this study we evaluated the antibacterial properties of silver ions implanted on AISI 304 stainless steel surfaces using low-energy doses against Salmonella Enteritidis and Listeria monocytogenes, two foodborne pathogens. AISI 304 stainless steel coupons were treated using energy of 2 and 4 keV for silver implantation and simulations were performed to estimate its dose distribution. Coupons containing silver ions were contaminated with S. Enteritidis and L. monocytogenes and incubated at 25 °C for 1 and 24 h. Results demonstrated that 4 keV treatment were able to reduce S. Enteritidis, but not L. monocytogenes. However, the 2 keV treatment showed significant reductions of both pathogens and the depth profiles of surfaces treated with 2 keV of energy showed 3.5×1016 silver atoms/cm² implanted in up to 5 nm from the stainless steel surface. Silver implanted on stainless steel using low-energy doses demonstrated antimicrobial properties against foodborne pathogens and this strategy can be used to reduce adhered cells and biofilm formation in food industries.
Keywords: bacterial adhesion, biofilm, foodborne pathogens, ion implantation, silver ions
Foodborne illnesses are considered an important health concern worldwide [1,2], consequently, strategies to reduce microbial contamination of food remain an important challenge in food industries. Equipment surfaces have been identified as sources of microbial contamination [3,4] and, among the most important foodborne pathogens able to adhere and form biofilms on stainless steel surfaces of food equipment and utensils are Salmonella spp. and L. monocytogenes [5-7]. Inside biofilms, these bacteria became more resistant to disinfectants and can cross-contaminate foods [8,9], increasing the risk of foodborne diseases.
Many equipment surfaces and utensils used in food processing are made of AISI 304 stainless steel due to its resistance to corrosion, ease of cleaning and low cost [10,11]. However, AISI 304 stainless steel surfaces do not have antibacterial properties [12,13], allowing the formation of biofilms, which can contaminate food. Silver is a well-known chemical element with antimicrobial properties [14,15], and when implemented on stainless steel surface can inactivate microorganisms and prevent biofilm formation. Studies using high and intermediate energy (>30 keV) to ion implementation showed that silver can be implemented in the first few micrometers of the stainless steel surface and this did not change any property of this material [16-19]. However, silver implementation studies using low-energy systems (0.1 to 10 keV) are few or unexplored. According to Echeverrigaray et al. [20] and Soares et al. [21], silver implanted with lower energy is capable of assuming equilibrium positions near the surface allowing spontaneous ionization. It facilitates ionic leaching through a shorter trajectory, making silver much more effective against bacterial agents. Furthermore, this technology can be carried out in industrial scale and can be more feasible than high-energy implantation.
Therefore, the aim of this study was to evaluate the effect against S. Enteritidis and L. monocytogenes of silver implanted on AISI 304 stainless steel surfaces using 2 keV and 4 keV energy doses.
2.1. Preparation of stainless steel coupons
AISI 304 stainless steel coupons were provided by Metalurgica Ralf Winter industry (Alvorada, Brazil). The coupons were cut in square shapes (2.0 cm × 2.0 cm × 0.2 cm) and were mechanically brushed and polished. After that, the stainless steel squares were immersed in acetone PA, treated by ultrasound for 30 min, and autoclaved at 121 °C for 15 min. Finally, coupons were dried at 60 °C for 2 h [22] and submitted to surface modification treatment.
2.2. Silver-ion implantation
Silver-ion implantation method used in this study was previously described by Echeverrigaray et al. [20] using an Ion Plating Diversified equipment. The main advantages of using this equipment are the possibility of ionic dose control, use of low temperatures, short-time duration, high reproducibility and low-energy implantation. Silver ions were implanted on all sides of the coupon surfaces. Briefly, stainless steel coupons were inserted in the vacuum reactor which was pumped down to a pressure of 1 × 10-5 Pa with a work pressure of 5 × 10-5 Pa. The silver implantation used was the pellet form with a purity of 99.9% provided by Kurt J. Lesker Company (United States). The process conditions of silver-ion implantation were 6 kV of voltage, 25 mA of emission current and 15.1 A of filament current during 60 min. Two treatments with different energy levels (BIAS) were tested: Treatment I used 4 keV and Treatment II used 2 keV.
2.3. Bacterial strains
We used two bacterial strains from the Laboratory of Food Microbiology and Food Control of the Institute of Food Science and Technology of Federal University of Rio Grande do Sul (ICTA/UFRGS), in Porto Alegre city, Southern Brazil. The first microorganism was S. Enteritidis (SE86), a gram-negative foodborne pathogen isolated from a cabbage responsible for a foodborne outbreak occurred in Rio Grande do Sul (RS). This microorganism was responsible for more than 90% of salmonellosis occurred in RS State from 1999 to 2013 [23]. The second microorganism was L. monocytogenes (J11), a gram-positive pathogen isolated from a bovine slaughterhouse in RS State, Brazil.
Before each experiment, S. Enteritidis was grown in Brain Hearth Infusion broth (BHI; Oxoid, Basingstoke, England) at 37 °C for approximately 18h and L. monocytogenes in BHI supplemented with 0.6% yeast extract (Oxoid, Basingstoke, England) incubated at 37 °C for approximately 30h. After incubation, both microorganisms were diluted using 0.1% peptone water (Oxoid, Basingstoke, England) until the final concentration of 106 CFU/mL and the bacterial suspension was used to artificially contaminate AISI 304 stainless steels coupons implanted with silver.
2.4. Surface cleaning treatment
Before microbiological testing, coupons were subjected to a surface cleaning treatment in order to remove contamination and reduce oxygen action. Stainless steel coupons with and without (controls) silver ion implanted were immersed in a cleaning solution (15% acetone PA and 85% solution NaOH 0.1 M), heated at 100 °C during 15 min and dried at ambient temperature.
2.5. Artificial contamination of coupons and evaluation of bacterial adhesion
Artificial contamination was carried out by immersing AISI 304 stainless steel coupons (Treatment I, Treatment II and controls) in 100 mL of bacterial suspensions containing 106 CFU/mL of S. Enteritidis or L. monocytogenes, separately. Coupons were kept in the bacterial suspension at 25 ± 1 °C under conditions of 1 and 24 h, to estimate the antimicrobial effect of silver on initial bacterial adhesion and on biofilm formation, respectively [24].
After each incubation time, the coupons were washed with 1 mL of sterile distilled water in order to remove the weakly adhered cells. After that, the coupons were immersed in 25 mL of 0.1% peptone water and treated in an ultrasonic bath for 10 min to detach adhered cells from AISI 304 coupon surfaces [25]. Decimal dilutions were carried out and 20 µL of each dilution were plated on agar plates using the drop method [26,27]. Triptic Soy Agar plates (TSA, Oxoid, England) were used to enumerate S. Enteritidis after incubation of 37 °C/18 h and TSA supplemented with 0.6% yeast extract to enumerate L. monocytogenes after incubation at 37 °C/30 h [7]. The reduction of initial bacterial adhesion (1h) and the biofilm formation (24h) were determined as antibacterial rate (AR) using the following equation [28]:
AR (%)=(100 (N1-N2))/N1 -(Eq.1)
where N1 is the number of bacteria adhered on untreated samples and N2 is the number of bacteria adhered on silver treated coupons.
2.6. Depth profile determination of treated surfaces
Simulation of trajectories and energy losses of implanted silver ions were carried out in stainless steel coupons using the software Stopping and Range of Ion in Matter (SRIM 2013) by Monte Carlo method (http://www.srim.org). The simulations were adjusted by low-energy standards of self-bias voltage acceleration of the silver ion beam with 2 keV (treatment that presented the highest AR for both tested bacteria) in stainless steel surface.
The silver quantitative chemical composition incorporated in stainless steel coupons was obtained by Rutherford Backscattering Spectrometry (RBS) using a 3 MV Tandem accelerator in order to produce a beam of mono-energetic helium (He) ions of high energy at 2 MeV with normal incidence and angle detection of backscattering of 165° [20]. Energy of backward scattered particles rise to detect spectrum and elemental composition of the material analyzed (atoms number). The atomic densities of Ag peak areas were estimated by standard Harwell bismuth (Bi) implanted into Si with 1.44 × 1016 atoms/ cm2. The areal density of Ag peak in the samples (atoms number per square centimeter) was calculated by Equation (2):
Q sample=Q standard . (A sample)/(N sample) . (N standard)/(A standard) . ((Z2 standard))/(Z2 sample)) -(Eq.2)
where Q is the amount of atoms/ cm2 of element, A is the area below the corresponding signal in the measured spectrum; N is the total number of incident ions and Z is the atomic number.
The surface chemical composition was obtained by glow discharge optical emission spectroscopy (GD-OES) using Horiba GD-Profiler 2 with a radio frequency (RF) power source of 15 W and a pressure of 650 Pa [20].
2.7. Statistical Analysis
Values of bacterial counts (CFU/mL) were converted to log CFU for statistical evaluation of the data obtained in bacterial tests. Three independent experiments were performed with duplicate repetitions. In order to analyze the data obtained in bacterial tests, variance analyses were performed using SPSS software version 21.0. The Tukey’s test was used to compare the averages and p<0.05 was considered significant.
Evaluation of bacterial reduction on AISI 304 stainless steel coupons
Coupons treated with 2 keV (Treatment II) demonstrated 51.1% and 66.7% of L. monocytogenes antibacterial rate (AR) in 1 and 24h, respectively (Fig. 1A). However, the reduction of this pathogen was not observed when coupons were treated with 4 keV of energy (Treatment I). AISI 304 stainless steel coupons submitted to both treatments demonstrated significant reductions of S. Enteritidis. The AR for this pathogen were similar after 1 and 24h (Fig. 1B), i.e. ~45% for the Treatment I and 68% for the Treatment II. The results demonstrated that Treatment II (2 keV) presented higher antibacterial rates for both pathogens tested, when compared to Treatment I (4 keV).
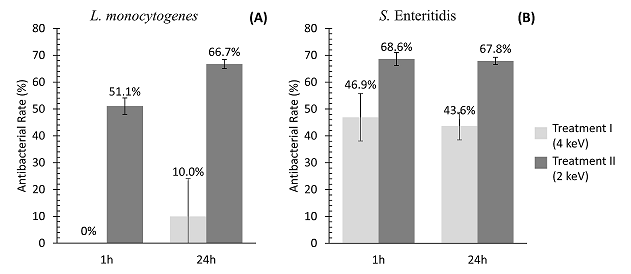
Figure 1. Antibacterial rate (AR) after 1 and 24h of L. monocytogenes (A) and S. Enteritidis (B) on AISI 304 stainless steel submitted to silver ion implantation at low-energies (2 and 4 keV).
The AR represents the percent reduction of bacterial cells adhered or inactivated on the silver treated coupons in relation to untreated coupons. Values are the means of three independent experiments.
The slightly lower antibacterial rate of L. monocytogenes (mainly at 1h) can be explained by its cellular composition. This microorganism is Gram-positive and its cell wall is composed by a thicker peptidoglycan layer, which can be more resistant against the silver ion penetration when compared to the thin cell wall of Gram-negative microorganisms as Salmonella [29-33].
According to Chen et al. [34] and confirmed by the present study, stainless steel implanted with silver can exhibit antibacterial activity against Gram-negative and Gram-positive bacteria. Chiang et al. [35] observed that AISI 316 stainless steel surfaces implanted with silver ions at a concentration of 0.03 % weight, using a vacuum induction melting furnace at 1050 °C for 5 minutes, were able to reduce 89.9% of an E. coli population. Liao et al. [36] found greater antibacterial rates (99.9%) for E. coli and Staphylococcus aureus, using 0.3 % wt Ag on AISI 304 stainless steel prepared in air induction furnace under a protective atmosphere at 1200 °C for 4 h. The lower antibacterial rates found in our study can be explained because we have tested other pathogens. Beyond that, our method for the implantation of silver on the stainless steel surface used low temperatures, short times and low energy than the methods used by Chiang et al. [35]and Liao et al. [36]. Similar results were found by Ni et al. [37] who observed good antibacterial activity against E. coli on AISI 420 stainless steel (77.7 %) using an ion implantation equipment with a dose of 2 × 1017 Ag/cm2. However, the antibacterial activity for S. aureus was lower (50%). In another study, Wan et al. [38] used an ion implantation equipment to implant silver on two materials (AISI 317 stainless steel and titanium). At an ion dose of 0.5 × 1017 Ag/cm2, the antibacterial effect for samples of AISI 317 and titanium treated with silver was 80 and 83%, respectively. When the silver ions were increased to 1.5 × 1017 Ag/cm2, antibacterial rates of 99.9% and 100% were obtained on the stainless steel and titanium samples, respectively. In the study of Echeverrigaray et al. [20] AISI 304 stainless steel coupons and AISI 316 stainless steel implanted with silver showed an initial bacterial reduction > 95% in comparison to the untreated samples for E. coli and S. aureus (with 1 × 1016 Ag/cm²). The bacterial reductions can be explained by the fact that silver, when released from the surface of stainless steel, acquires the ionized form and is able to bind to proteins of the bacterial cells. This process possibly damages the structure of the cell wall, causing the death of cells. However, some studies have shown that bacterial colonies can be formed on silver-treated surfaces during longer incubation periods [35]. Therefore, if the treated stainless steel surface is used in food industries or hospitals, it is also important to use appropriate cleaning and disinfection procedures to avoid the biofilm formation and the presence of pathogenic bacteria on the surfaces.
Depth profile of chemical composition using 2 keV of energy treatment
Figure 2 shows that the silver ionic implantation using 2 keV reached ions incorporation of up to 5 nm from the surface and the highest concentration was approximately 1.7 nm deep. Novello [39] also analyzed the implantation of silver ions using different energy conditions. This author demonstrated that with treatment of 200 keV, silver was implanted at a depth of 25 nm from the surface, whereas in samples treated with 70 keV, silver was implanted at 15 nm from the surface. The surface at which the silver ions were implanted with 70 keV demonstrated higher antimicrobial affect, because the ions were implanted closer to the surface, making possible a faster release of silver. According to Echeverrigaray et al. [20], silver implanted with lower energy assumes position of equilibrium close to the surface and ionizes spontaneously, facilitating the leaching of ions and making shorter the trajectories.
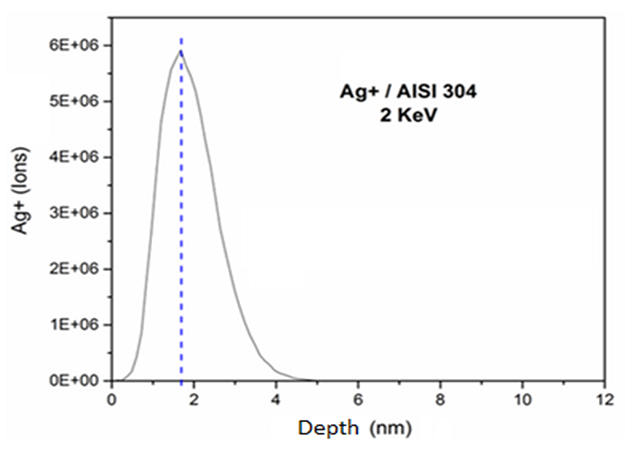
Figure 2. Simulated ion ranges of the 2 keV silver implanted on AISI 304 stainless steel.
With the results of the GD-OES analysis (Figure 3), it was possible to observe that coupons presented a dose of 3.5 × 1016 Ag/cm2 (6.3 µg/cm²) in AISI 304 stainless steel after 24 hours. The GD-OES results demonstrated that the elements of stainless steel were iron and chromium. In addition, the curves of the elements do not show an abrupt growth, which indicates that the silver was implanted in the outermost layer of the stainless steel surface. It was also verified the presence of oxygen on the stainless steel surface, which corresponds to the oxide layer that is formed by exposure to the environment.
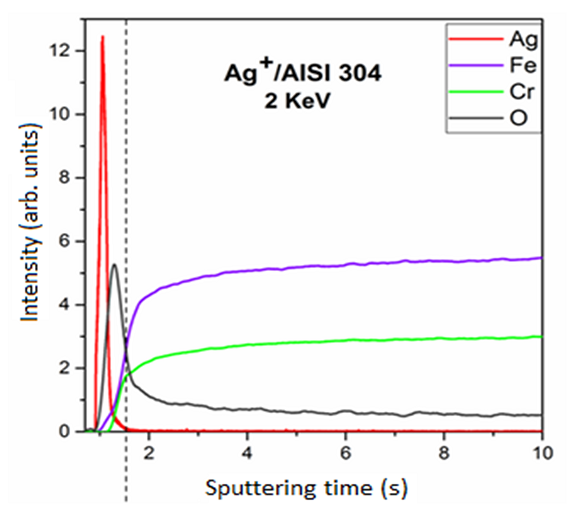
Figure 3. Chemical configuration profile in-depth by GD-OED spectrum on AISI 304 with 2 keV.
The implant of silver on AISI 304 stainless steel was effective in reducing S. Enteritidis and L. monocytogenes adhered and forming biofilms on the surfaces. The silver implant on the surface using low energy (2 keV) reached only a few nanometers of the surface and showed greater antimicrobial rates against the pathogens tested when compared to the application of 4 keV of energy. This technique, besides being economical due to low energy and small amounts of silver (6.3 µg/cm2) implemented, presents a high degree of reproducibility. This demonstrates a potential use on stainless steel surfaces, possibly bringing benefits to food industries in preventing food contamination by Salmonella and L. monocytogenes. However, it is important to highlight that other food pathogens should be tested, as well as other silver implementation systems on industrial scale should be further studied in order to obtain further information about antimicrobial properties of silver implanted on stainless steel.
The authors thanks CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAUFRGS (Fundação de Apoio da Universidade do Rio Grande do Sul) and CNPq (Conselho Nacional de Desenvolvimento Científico).
Authors' contributions
LPS and ECT designed the research. RZ performed the microbiological experiments. RZ and CIK analyzed the data and wrote the draft of the manuscript. TPSZ and CA performed the silver implantation and the chemical composition analyses. LPS and ECT revised the manuscript and supervised the project. All authors approved the submitted version.
Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Dopfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ (2015) World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. Plos Med 12 (12). doi:10.1371/journal.pmed.1001921 PMid:26633831
View Article PubMed/NCBIMarder EP, Griffin PM, Cieslak PR, Dunn J, Hurd S, Jervis R, Lathrop S, Muse A, Ryan P, Smith K, Tobin-D'Angelo M, Vugia DJ, Holt KG, Wolpert BJ, Tauxe R, Geissler AL (2018) Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food - Foodborne Diseases Active Surveillance Network, 10 US Sites, 2006-2017. Morb Morbid Mortal W 67 (11):324-328. doi:10.15585/mmwr.mm6711a3 PMid:29565841
View Article PubMed/NCBINewman KL, Bartz FE, Johnston L, Moe CL, Jaykus LA, Leon JS (2017) Microbial Load of Fresh Produce and Paired Equipment Surfaces in Packing Facilities Near the US and Mexico Border. J Food Protect 80 (4):582-589. doi:10.4315/0362-028x.Jfp-16-365 PMid:28271928
View Article PubMed/NCBIFaille C, Cunault C, Dubois T, Benezech T (2018) Hygienic design of food processing lines to mitigate the risk of bacterial food contamination with respect to environmental concerns. Innov Food Sci Emerg 46:65-73. doi:10.1016/j.ifset.2017.10.002
View ArticleTondo EC, Machado TRM, Malheiros PD, Padrao DK, de Carvalho AL, Brandelli A (2010) Adhesion and Biocides Inactivation of Salmonella on Stainless Steel and Polyethylene. Braz J Microbiol 41 (4):1027-1037. doi:Doi 10.1590/S1517-83822010000400022
View ArticleCarpentier B, Cerf O (2011) Review - Persistence of Listeria monocytogenes in food industry equipment and premises. Int J Food Microbiol 145 (1):1-8. doi:10.1016/j.ijfoodmicro.2011.01.005 PMid:21276634
View Article PubMed/NCBICasarin LS, Brandelli A, Casarin FD, Soave PA, Wanke CH, Tondo EC (2014) Adhesion of Salmonella Enteritidis and Listeria monocytogenes on stainless steel welds. Int J Food Microbiol 191:103-108. doi:10.1016/j.ijfoodmicro.2014.09.003 PMid:25261827
View Article PubMed/NCBINguyen HDN, Yang YS, Yuk HG (2014) Biofilm formation of Salmonella Typhimurium on stainless steel and acrylic surfaces as affected by temperature and pH level. LWT-Food Sci Technol 55 (1):383-388. doi:10.1016/j.lwt.2013.09.022
View ArticleZhao XH, Zhao FH, Wang J, Zhong NJ (2017) Biofilm formation and control strategies of foodborne pathogens: food safety perspectives. Rsc Adv 7 (58):36670-36683. doi:10.1039/c7ra02497e
View ArticleShi XM, Zhu XN (2009) Biofilm formation and food safety in food industries. Trends Food Sci Tech 20 (9):407-413. doi:10.1016/j.tifs.2009.01.054
View ArticleCasarin LS, Casarin FD, Soares TP, Aguzzoli C, Figueroa CA, Soares GV, Brandelli A, Tondo EC (2016) Effect of Plasma Nitriding Surface Modification on the Adhesion of Food Pathogens to Stainless Steel AISI 316 and AISI 304. J Food Safety 36 (3):341-347. doi:10.1111/jfs.12249
View ArticleMonteiro DR, Gorup LF, Takamiya AS, Ruvollo AC, Camargo ER, Barbosa DB (2009) The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Ag 34 (2):103-110. doi:10.1016/j.ijantimicag.2009.01.017 PMid:19339161
View Article PubMed/NCBIArciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW (2012) Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials 33 (26):5967-5982. doi:10.1016/j.biomaterials.2012.05.031 PMid:22695065
View Article PubMed/NCBIGaleano B, Korff E, Nicholson WL (2003) Inactivation of vegetative cells, but not spores, of Bacillus anthracis, B. cereus, and B. subtilis on stainless steel surfaces coated with an antimicrobial silver- and zinc-containing zeolite formulation. Appl Environ Microb 69 (7):4329-4331. doi:10.1128/Aem.69.7.4329-4331.2003 PMid:12839825
View Article PubMed/NCBIKampmann Y, De Clerck E, Kohn S, Patchala DK, Langerock R, Kreyenschmidt J (2008) Study on the antimicrobial effect of silver-containing inner liners in refrigerators. J Appl Microbiol 104 (6):1808-1814. doi:10.1111/j.1365-2672.2008.03727.x PMid:18341560
View Article PubMed/NCBIFeng HY, Yu ZL, Chu PK (2006) Ion implantation of organisms. Mat Sci Eng R 54 (3-4):49-120. doi:10.1016/j.mser.2006.11.001
View ArticleWan YZ, Raman S, He F, Huang Y (2007) Surface modification of medical metals by ion implantation of silver and copper. Vacuum 81 (9):1114-1118. doi:10.1016/j.vacuum.2006.12.011
View ArticleJain IP, Agarwal G (2011) Ion beam induced surface and interface engineering. Surf Sci Rep 66 (3-4):77-172. doi:10.1016/j.surfrep.2010.11.001
View ArticleFang F, Kennedy J, Dhillon M, Flint S (2015) Antibacterial effect of silver nanofilm modified stainless steel surface. Int J Mod Phys B 29 (10-11). doi:10.1142/S0217979215400135
View ArticleEcheverrigaray FG, Echeverrigaray S, Delamare APL, Wanke CH, Figueroa CA, Baumvol IJR, Aguzzoli C (2016) Antibacterial properties obtained by low-energy silver implantation in stainless steel surfaces. Surf Coat Tech 307:345-351. doi:10.1016/j.surfcoat.2016.09.005
View ArticleSoares TP, Garcia CSC, Roesch-Ely M, da Costa MEHM, Giovanela M, Aguzzoli C (2018) Cytotoxicity and antibacterial efficacy of silver deposited onto titanium plates by low-energy ion implantation. J Mater Res 33 (17):2545-2553. doi:10.1557/jmr.2018.200
View ArticleRossoni EMM, Gaylarde CC (2000) Comparison of sodium hypochlorite and peracetic acid as sanitising agents for stainless steel food processing surfaces using epifluorescence microscopy. Int J Food Microbiol 61 (1):81-85. doi:Doi 10.1016/S0168-1605(00)00369-X 00369-X
View ArticleTondo EC, Ritter, A. C., & Cassarin, L. S. (2015) Involvement foodborne outbreaks, risk factors and options to control Salmonella Enteretidis SE86: an important food pathogens in Southern Brazil. In: CB H (ed) Salmonella. Nova Publishers, New York, pp 175-191
Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR (2003) Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol 85 (3):227-236. doi:10.1016/S0168-1605(02)00540-8 00540-8
View ArticleSinde E, Carballo J (2000) Attachment of Salmonella spp. and Listeria monocytogenes to stainless steel, rubber and polytetrafluorethylene: the influence of free energy and the effect of commercial sanitizers. Food Microbiol 17 (4):439-447. doi:DOI 10.1006/fmic.2000.0339
View ArticleMiles AA, Misra SS, Irwin JO (1938) The estimation of the bactericidal power of the blood. J Hyg (Lond) 38 (6):732-749. doi:10.1017/s002217240001158x PMid:20475467
View Article PubMed/NCBISilva N, Junqueira, V. C. A., Silveira, N. F. A, Taniwaki, M. H., Santos, R. F. S., Gomes, R. A. R. (2010) Manual de Métodos de Análise Microbiológica de Alimentos e Água Livraria Varela, São Paulo
Lin LH, Chen SC, Wu CZ, Hung JM, Ou KL (2011) Microstructure and antibacterial properties of microwave plasma nitrided layers on biomedical stainless steels. Appl Surf Sci 257 (17):7375-7380. doi:10.1016/j.apsusc.2011.01.065
View ArticleRai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27 (1):76-83. doi:10.1016/j.biotechadv.2008.09.002 PMid:18854209
View Article PubMed/NCBIGreulich C, Braun D, Peetsch A, Diendorf J, Siebers B, Epple M, Koller M (2012) The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. Rsc Adv 2 (17):6981-6987. doi:10.1039/c2ra20684f
View ArticleKumar-Krishnan S, Prokhorov E, Hernandez-Iturriaga M, Mota-Morales JD, Vazquez-Lepe M, Kovalenko Y, Sanchez IC, Luna-Barcenas G (2015) Chitosan/silver nanocomposites: Synergistic antibacterial action of silver nanoparticles and silver ions. Eur Polym J 67:242-251. doi:10.1016/j.eurpolymj.2015.03.066
View ArticleMai-Prochnow A, Clauson M, Hong JM, Murphy AB (2016) Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep-Uk 6. doi:10.1038/srep38610 PMid:27934958
View Article PubMed/NCBIBrobbey KJ, Haapanen J, Makela JM, Gunell M, Eerola E, Rosqvist E, Peltonen J, Saarinen JJ, Tuominen M, Toivakka M (2019) Effect of plasma coating on antibacterial activity of silver nanoparticles. Thin Solid Films 672:75-82. doi:10.1016/j.tsf.2018.12.049
View ArticleChen M, Yu QS, Sun HM (2013) Novel Strategies for the Prevention and Treatment of Biofilm Related Infections. Int J Mol Sci 14 (9):18488-18501. doi:10.3390/ijms140918488 PMid:24018891
View Article PubMed/NCBIChiang WC, Tseng IS, Moller P, Hilbert LR, Tolker-Nielsen T, Wu JK (2010) Influence of silver additions to type 316 stainless steels on bacterial inhibition, mechanical properties, and corrosion resistance. Mater Chem Phys 119 (1-2):123-130. doi:10.1016/j.matchemphys.2009.08.035
View ArticleLiao KH, Ou KL, Cheng HC, Lin CT, Peng PW (2010) Effect of silver on antibacterial properties of stainless steel. Appl Surf Sci 256 (11):3642-3646. doi:10.1016/j.apsusc.2010.01.001
View ArticleNi HW, Zhang HS, Chen RS, Zhan WT, Huo KF, Zuo ZY (2012) Antibacterial properties and corrosion resistance of AISI 420 stainless steels implanted by silver and copper ions. Int J Min Met Mater 19 (4):322-327. doi:10.1007/s12613-012-0558-6
View ArticleWang HH, Manuzon M, Lehman M, Wan K, Luo HL, Wittum TE, Yousef A, Bakaletz LO (2006) Food commensal microbes as a potentially important avenue in transmitting antibiotic resistance genes (vol 254, pg 228, 2006). Fems Microbiol Lett 255 (2):328-328. doi:10.1111/j.1574-6968.2006.00138.x
View ArticleNovello JCL (2012) Implantação de íons de prata em aço inoxidável e infecção fágica para o controle de adesão e formação de biofilmes bacterianos na indústria de alimentos. Doutorado em Ciência e Tecnologia de Alimentos, Universidade Federal de Viçosa, Viçosa, MG. doi:locus.ufv.br/handle/123456789/466
