Dongshun Zhou,( Email: Zhoudongshun@163.com)
Renzhong Wan, (Email: wrzh63@163.com)
Yuping Jia, (Email: jiayupingygs@163.com), Tel: +86-531-82919703/67790612
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 6
Page No: 840-845
Dongshun Zhou,( Email: Zhoudongshun@163.com)
Renzhong Wan, (Email: wrzh63@163.com)
Yuping Jia, (Email: jiayupingygs@163.com), Tel: +86-531-82919703/67790612
Yuping Jia1*, Shuqian Lin 2, 3, Kai Chen1, Qingwen Jia1, Sha Liu1, Xianli Zhang1, Meichao Lu1, Renzhong Wan 2*, Dongshun Zhou 4*
Yu-Li Jiang(renren108@126.com)
Ling Han(linghan36@163.com)
Yuping Jia, Shuqian Lin, Kai Chen, Qingwen Jia, Sha Liu, Xianli Zhang, Meichao Lu, Renzhong Wan, Dongshun Zhou, The Anti-Hyperplasia of Mammary Gland Effect of Chinese Traditional Medicine compound RPXT in Rats (2019)Journal of Food Science & Technology 4(6), 840-845
Rupixiao Tablets (RPXT) is a Chinese Traditional Medicine compound and comprises of 15 Chinese medicines, but the effect of anti-mammary gland hyperplasia, toxicity and mechanism are not clear. We investigated the protective effect of RPXT on hyperplasia of mammary gland (HMG) induced by estrogen and progestogen in SD rats. The rats were randomly divided into three groups (n = 10), including normal control group, HMG model group and RPXT treatment group. The toxicity study of RPXT on HMG rats was performed at 500 mg/kg/day by i.g. for 35 consecutive days. Changes of nipple height and diameter, serum sex hormones levels and organ indexes, pathologic changes of mammary gland, heart, liver, spleen, kidney and lung, hematological and biochemical analysis were measured or observed to evaluate the anti-HMG effect and toxicology of RPXT. The expression of AP-2α, P53 and caspase-3 in mammary gland of rats were analyzed by western blot. The results indicated that RPXT could remarkably decrease nipple height and diameter, uterus index and E2 concentration of HMG rat serum, and no toxicity at dose of 500 mg/kg/day. AP-2α, P53 and caspase-3 expression in mammary gland was significantly decreased in mammary gland of RPXT -treated HMG rats. The whole results indicated that RPXT has protective and therapeutic effects on HMG rats, and is a promising Chinese Traditional Medicine for human mammary cancer prevention which is related to caspase-3, AP-2α and P53 expression.
Key words: Chinese Traditional Medicine compound; anti-hyperplasia of mammary gland; toxicity; RPXT
Hyperplasia of mammary gland (HMG) is a kind of pathological hyperplasia of lobules of mammary gland, induced by the balance disorder of estrogen and progesterone in the middle-aged women. As a risk of causing mammary carcinoma, the morbidity of HMG is increasing nowadays [1-2]. So, it is important to discovery drugs that are more convenient, effective, and have fewer side effects for the treatment of HMG, and to explore the anti-HMG mechanisms of these drugs.
Rupixiao Tablets (RPXT) is a Chinese Traditional Medicine compound and comprises of 15 Chinese medicines and the main ingredients of RPXT are Cervi Cornu, Taraxaci Herba, Laminariae seu Eckloniae Thallus, Trichosanthis Radix, Spatholobi Caulis;, Notoginseng Radix et Rhizoma, Paeoniae Radix Rubra, Sargassum, Rhapontici Radix, Aucklandiae Radix, Scrophulariae Radix, Moutan Cortex, Prunellae Spica, Forsythiae Fructus, Carthami Flos. It acts to soften hardness, dissipate stasis, activate blood, relieve pain, clear heat and remove toxin [3], but the effect of anti-mammary gland hyperplasia, toxicity and mechanism are not clear.
In the present study, we evaluated the anti-HMG effects and the toxicity of RPXT at 500 mg/kg/day in HMG rats which were induced by estrogen and progestogen. The expression of caspase-3, AP-2α and P53 in mammary gland was detected by western blot.
2.1 Chemicals
Rupixiao Tablets (RPXT) was obtained from Liaoning Herbpex pharmaceulical (Group) Co., Ltd. Estrogen benzoate injection was purchased from Ningbo Second Hormone Factory. Progestogen injection was purchased from Shanghai general pharmaceutical Co., Ltd. Progesterone (P), Estradiol (E2), Prolactin (PRL) and Testosterone (T) ELISA kits were purchased from Shanghai Hengyuan Biological Technology Co., Ltd. All other regents and solvents were of analytical grade.
2.1 Animals and treatments
Virgin female Sprague Dawley rats weighing 180–220 g was supplied by Vital River Laboratory Animal Technology Co. Ltd. Experimental protocols followed standards and policies of Shandong Academy of Pharmaceutical Sciences Animal Care and Use Committee. They were allowed for acclimation for a week before use. The rats were housed in plastic cages with room temperature of 24±1°C with a relative humidity of 55±5% under a 12 h light-dark cycle and provided with rodent chow and water ad libitum.
2.3 Animal model and experimental groups
The rats were randomly divided into three groups (n = 10), rats in the normal control group were administered with normal saline intramuscularly, rats in other groups were treated with estrogen (0.5 mg/kg) intramuscularly for 30 days, and followed with progestogen (5 mg/kg) for another 5 days to induce HMG model [4-5]. From the 36th day, rats were treated with RPXT (500 mg/kg) in RPXT group, normal saline in the normal control group and HMG model group by gavage for 35 days.
2.4 Body weight, food intake, nipple height and diameter, and organ indexes
Body weight, food consumption and nipple height and diameter of HMG rats were recorded on the first day of administration, and then once a week thereafter and until termination. Organ indexes (uterus, spleen and thymus) were calculated as uterus, spleen or thymus weight divided by body weight. Organ index was calculated by the following formulae: Organ index﹦(W organ×10)/ W body. Worgan and Wbody stand for the average weights of uterus/spleen/thymus and body of the rats [6].
2.5 ELISA
At the end of experiments, blood samples were centrifuge at 3000 rpm for 10 min at 4°C, the levels of sex hormone in blood serum was observed before the rats were sacrificed. E2, T, PRL and P concentrations in the HMG rat serum were measured by enzyme-linked immunosorbent assay (ELISA) kits according to the procedures recommended by the manufacturer (Shanghai Hengyuan Biological Technology Co., Ltd, China).
2.6 Hematological assay and serum biochemistry
Blood samples were collected for analysis of hematology and serum biochemistry at the end of experiments. Hematological parameters included white blood cell count (WBC), red blood cell (RBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), platelet count (PLT), lymphocytes (LYM), monocytes (MON), neutrophilic granulocytes (NEUT), eosinophils (EOS), basophilic granulocyte (BAS). The clinical chemistry parameters included alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), blood urea nitrogen (BUN), triglyceride (TG), total protein (TP), creatine kinase (CK), total bilirubin (TBIL), albumin (ALB) and creatinine (CREA).
2.7 Histological observations
Tissues of mammary gland, heart, liver, spleen, kidney and lung from all groups were fixed in 10% buffered formalin, embedded in paraffin, sectioned into 4 μm pieces and stained with Hematoxylin-Eosin (H-E) and examined using optical microscopy.
2.8 Western blot
The expression of AP-2α, P53 and caspase-3 in mammary gland of RPXT group and HMG model group rats were analyzed by western blot. Total protein of them was transferred to Poluvinglidene Fluoride (PVDF) membrane (Millipore, USA). AP-2a, P53 and caspase-3 (1:1000, Cell Signaling Technology, USA) antibodies were added and incubated for 1 h at room temperature and then detected the corresponding HRP-labelled secondary antiserum (1:4000, Santa Cruz Biotechnology, INC). A β-actin (1:5000, Santa Cruz Biotechnology, INC) antibody was used for loading control [6].
2.9 Statistical analysis
Data were analyzed by Student’s two-tailed t test or Microsoft Excel and presented as the mean ± SD. Significant difference is indicated as * p < 0.05 and ** p < 0.01.
3.1 Effect of RPXT on nipple height and diameter in rats
Figure 2 shows the Effect of RPXT on nipple height and diameter in rats. From the figure, the height and diameter of nipples (Left 2 and Right 2) of RPXT group and normal control group were obviously reduced compared with HMG model group (P< 0.01) during the intragastric administration for 35d.
Fig.1 Height and diameter of left and right nipples in different groups.
3.2 Effects of RPXT on uterus, spleen and thymus index in rats
Figure 2 shows the Effect RPXT on uterus, spleen and thymus index in rats. From the figure, uterus index of RPXT group were significantly decreased (P<0.01) compared with HMG model group. Spleen index and thymus index of RPXT group had no significant difference (P>0.05) compared with HMG model group. These results indicate that RPXT maybe play anti-HMG function by decreasing uterus index to inhibit hypertrophy and hyperplasia of the uterus.
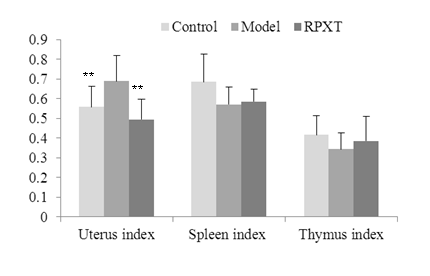
Fig.2 Effects on uterus, thymus and spleen index in different groups.
3.3 Effect of RPXT on serum sex hormone levels in HMG model rats
Figure 3 shows the Effect of RPXT on serum sex hormone levels in HMG model rats. Compared with normal control group, HMG model rats serum estradiol (E2) concentration was obviously increased (P<0.01); progesterone (P) and testosterone (T) concentration was significantly decreased (P<0.05); prolactin (PRL) had no significant difference (P>0.05). E2 concentration of RPXT group was decreased significantly compared with HMG model group ((P<0.01), but T, P and PRL concentration of RPXT group had no significant difference (P>0.05).
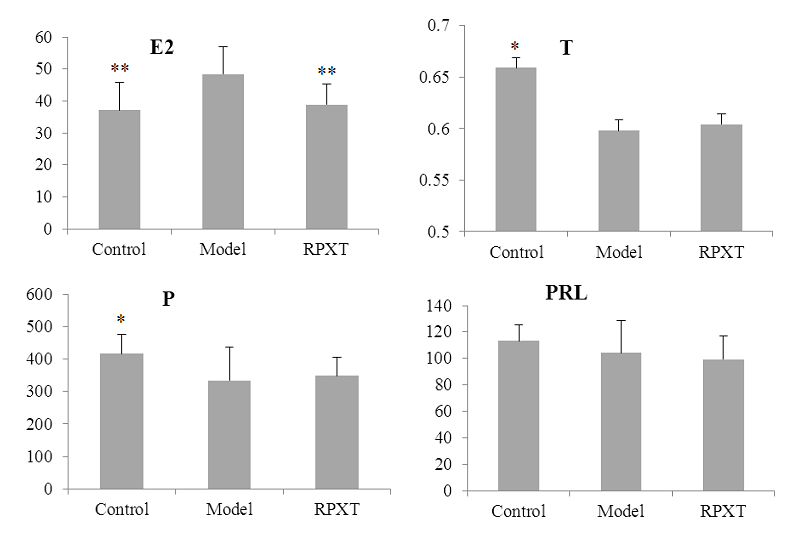
Fig.3 Serum sex hormone concentration in different groups.
3.4 Effect of RPXT on mammary gland tissue
Figure 4 shows the Effect of RPXT on mammary gland tissue. From the figure, Pathomorphology examination of mammary gland tissue in normal control group showed no proliferative lesions, no mammary ducts ectasia and no expansion of mammary lumens (Fig.4.A). The rat’s mammary gland tissue in HMG model group had histological abnormalities, including significantly proliferative lesions, mammary ducts ectasia expansion of mammary lumens; acinus and lobules significantly increased (Fig. 4. B). Compared with HMG model group, hyperplasia of mammary gland in RPXT group was obviously alleviated, and the number of acinus was markedly decreased (Fig. 4.C). These results indicate that RPXT has therapeutic effect on HMG rats induced by estrogen and progestogen.
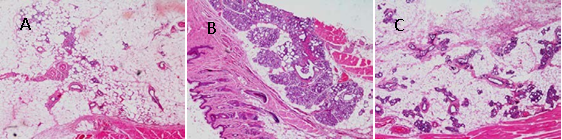
Fig.4 Histological images of mammary gland tissue (original magnification, 40×). (A) Normal control group; (B) HMG model group; (C) RPXT group
3.5 Toxicity of RPXT in HMG rats
The toxicity study of RPXT group and HMG model group rats include body weight, food intake, histopathological analysis, hematological and biochemical analysis. Figure 5 shows the effect of RPXT on the food intakes and body weights of HMG rats with 500 mg/kg. From the figure, the body weights and food intakes of the RPXT-treated HMG rats (500 mg/kg/day) had no significant difference compared with HMG model rats.
Figure 6 shows the histopathologic analysis by H&E staining of RPXT-treated HMG rats and HMG model rats. From the figure, the five organs (heart, liver, spleen, kidney and lung) had no obvious morphologic difference between RPXT group rats and HMG model group rats.
There was no effect on hematological parameters and serum biochemical parameters between RPXT group and HMG model group, including WBC, RBC, HGB, HCT, MCV,MCH, PLT, LYM, MON, NEUT, EOS, BAS; and ALT, AST, ALP, ALB, BUN, TG, TP, CK, TBIL and CREA levels (P>0.05)(data not shown). These results indicated that RPXT has no toxic effect for HMG rats at dose of 500 mg/kg/day.
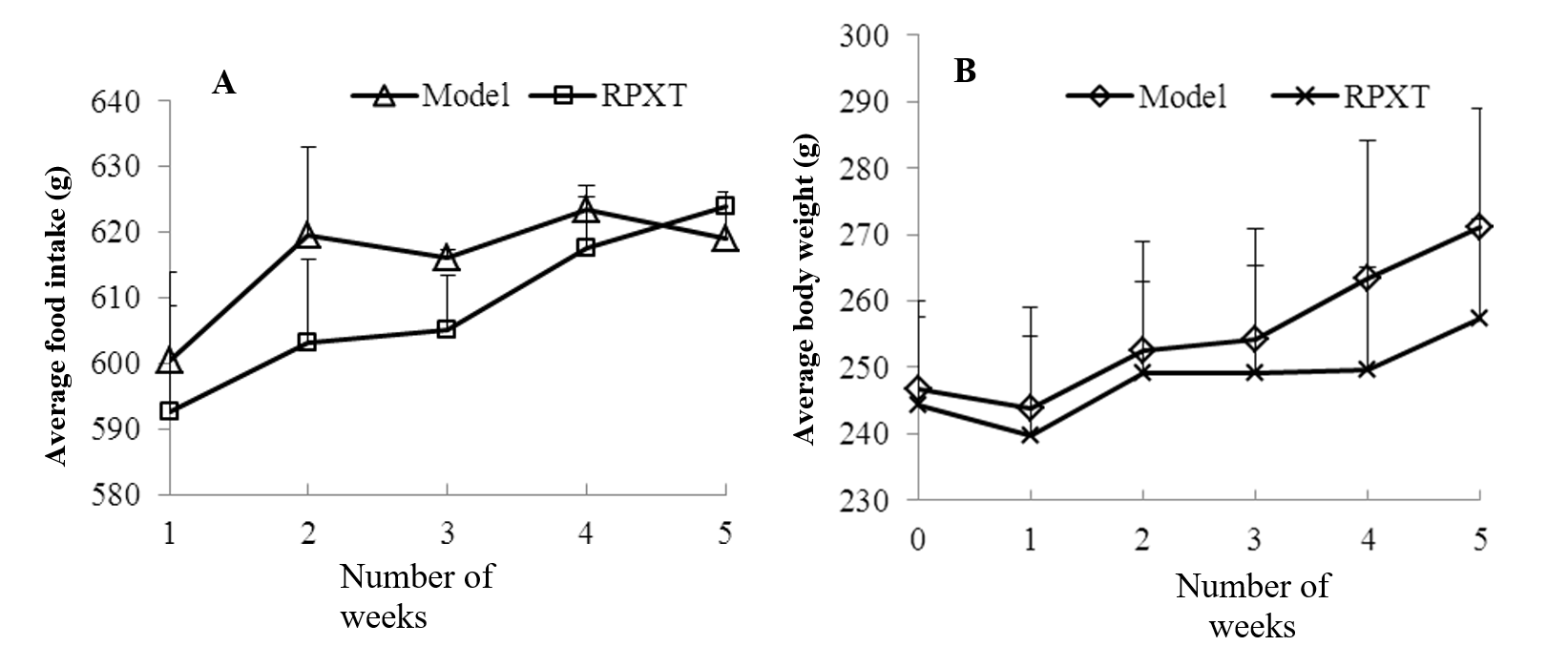
Fig. 5 Effects of RPXT on the food intakes (A) and body weights (B) of HMG rats with 500 mg/kg.
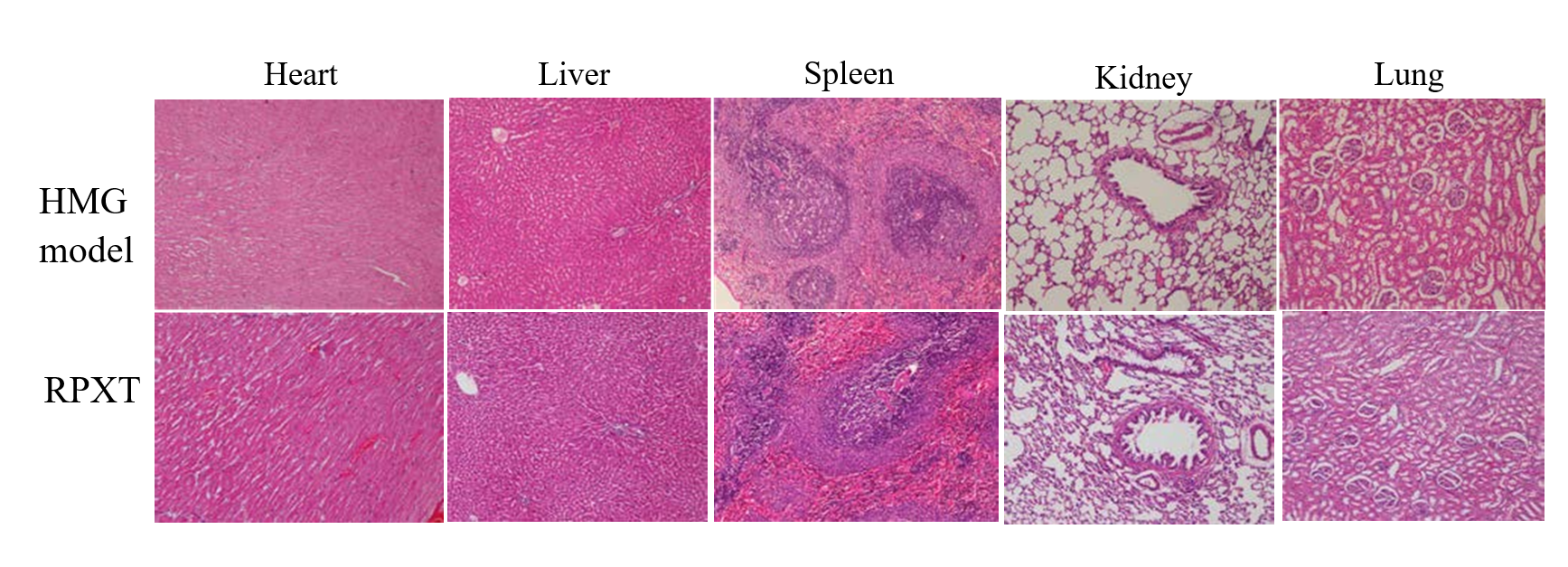
Fig.6 Histopathologic analysis by H&E staining of organs (heart, liver, spleen, kidney and lung) of RPXT-treated HMG rats compared with HMG model rats.
3.6 Western blot analysis of mammary gland tissues
Figure 7 shows the expression of AP-2α, P53 and caspase-3 in mammary gland tissues of HMG model and RPXT-treated rats by western blot. From the figure, AP-2α, P53 and caspase-3 expression in mammary gland was significantly decreased in mammary gland of RPXT -treated HMG rats.
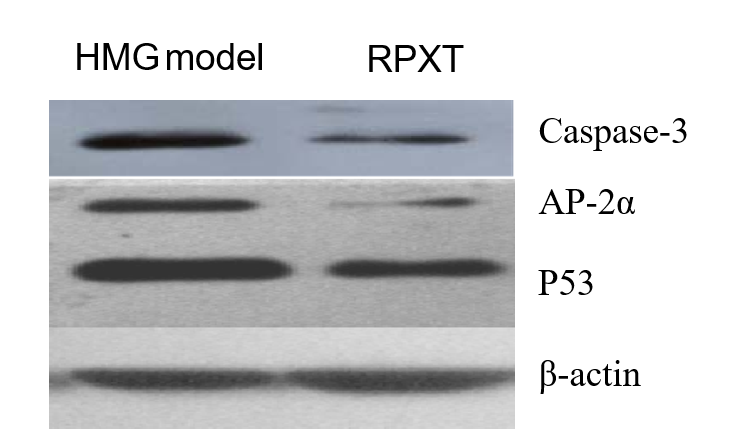
Fig.7 Western blot analysis of mammary gland tissues. The expression of AP-2α, P53 and caspase-3 was analyzed by Western blot in mammary gland tissues of HMG model and RPXT-treated rats.
This study provides evidence that RPXT has protective and therapeutic effects on HMG rats and no toxicity at dose of 500mg/kg. Uterus and mammary gland are the main target organ of estrogen [7]. RPXT could decrease E2 concentration, uterus index and hyperplasia of mammary gland of HMG rats, so RPXT should have protective effect on uterus and mammary gland of HMG rat and play the role of anti-HMG by regulating hormone level. The heights and diameters of nipples, volume and number of mammary lobules and acinus in RPXT group rats remarkably decreased. The results reveal that the mammary gland of RPXT group rats recovered well and RPXT has therapeutic effect on HMG rats induced by estrogen and progestogen.
Caspase-3 is involved in carcinogenesis and progression of breast cancer,which suggests that increased expression of caspase-3 is common events in breast cancer. The expression of caspase-3 protein in invasive duct carcinoma (IDC), ductal carcinoma in situ (DCIS) and HMG was higher than that in tumor adjacent tissues and that in IDC and DCIS was higher than that in HMG[8]. The expression of caspase-3 in breast cancer was significantly higher than in breast benign hyperplasia and normal breast, and that of in breast benign hyperplasia was higher than that in normal breast[9]. AP-2 and P53 family members have different function at various developmental and lifetime periods. P53 family is related to the function and expression of AP-2 family members during different stages of normal development or disease processes[10]. AP-2α is a member of AP-2 family and required for normal growth and morphogenesis and an important for cellular functions such as proliferation, apoptosis and differentiation in the mammary epithelium under physiological conditions[11- 12]. The western blot results indicate that the expression of caspase-3, AP-2α and P53 in mammary gland of HMG model group was higher than that in mammary gland of RPXT group. The reasons may be that the degree of HMG reduced by RPXT is related to the expression of caspase-3, AP-2α and P53 in mammary gland. Low-expression of AP-2α and P53 caused by RPXT in mammary gland reduce cell proliferation, or lead to cell cycle arrest and then suppressed mammary gland growth and morphogenesis. The exact anti-HMG effect mechanism of RPXT need more studies to clarity.
RPXT has protective and therapeutic effect, no toxic effect on HMG rats, and is a promising Chinese Traditional Medicine for human mammary cancer prevention which is related to caspase-3, AP-2α and P53 expression. Further studies would focus on the exact anti-HMG effect mechanism of RPXT.
This work was supported by Public service platform for preclinical evaluation of innovative drugs (CXLC161906) and Special Fund for Agro-scientific Research in the Public Interest (201303040-10).
Bennett IC, Cufffrey MC, Mccattrey E. Serum estradiol in women with and without breast disease. Br. J. Cancer. 1990; 61: 142-147. PMid:2393409
View Article PubMed/NCBIWang LS, Zhao DQ, Lin D. The anti-hyperplasia of mammary gland effect of Thladiantha dubia root ethanol extract in rats reduced by estrogen and progestogen. J. Ethnopharmacol. 2011; 134: 136-140. PMid:21134436
View Article PubMed/NCBIChinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China, China Medical Science Press: Beijing, China, Volume I; 2015:1454-1455.
Rao JC, Li LZ, Chen YS. The preparation and pathological type of HMG animal model. Chinese Journal of Pathophysiology. 1992; 8: 671.
Milliken EL, Ameduri RK, Landis MD. Ovarian hyperstimulation by LH leads to mammary gland hyperplasia and cancer predisposition in transgenic mice. Endocrinology. 2002; 143: 3671-3680. PMid:12193583
View Article PubMed/NCBIJia Y, Liu X, Jia Q, Zhang W, Sun C, Yuan D, Zhang H, Jiang E, Zhou D. The anti-hyperplasia of mammary gland effect of protein extract HSS from Tegillarca granosa. Biomed Pharmacother. 2017; 85:1-6. PMid:27930972
View Article PubMed/NCBIZhou JH. Immune pharmacology of Traditional Chinese medicine. People's Medical Publishing House: Beijing, China; 1994; 47.
Wang JJ,Sun BC. Expression of caspase-3 and caspase-9 and their significance in breast cancer. J Clin Exp Pathol. 2012; 28: 378-381
Dong PX. Research on the Expression of Survivin, VEGF and Caspase-3 in Breast Cancer. Clinical medical engineering. 2014; 21:697-698.
Li H, Watts GS, Oshiro MM, Futscher BW, Domann FE. AP-2a and AP-2c are transcriptional G.S. targets of p53 in human breast carcinoma cells, Oncogene. 2006; 25: 5405-5415. PMid:16636674
View Article PubMed/NCBIFriedrichs N, Steiner S, Buettner R, Knoepfle G. Immunohistochemical expression patterns of AP2alpha and AP2gamma in the developing fetal human breast, Histopathology. 2007; 51:814-823. PMid:18042070
View Article PubMed/NCBIJäger R, Friedrichs N, Heim I, Büttner R, Schorle H. Dual role of AP-2gamma in ErbB-2-induced mammary tumorigenesis, Breast Cancer Res Treat, 2005; 90: 273-280. PMid:15830141
View Article PubMed/NCBI