Dina Mostafa Mohammed, Ph.D.
Affiliation and contact details: Assistant Researcher, Nutrition and Food Science, National Research Centre, Egypt. Tel.: 00201003715090
E-mail: dina_ganna@yahoo.com
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 5
Page No: 440-449
Dina Mostafa Mohammed, Ph.D.
Affiliation and contact details: Assistant Researcher, Nutrition and Food Science, National Research Centre, Egypt. Tel.: 00201003715090
E-mail: dina_ganna@yahoo.com
Afaf Ezzat 1, Abdou Osman Abdelhamid2, and Dina Mostafa Mohammed1
1Department of Nutrition and Food Science, National Research Centre, Dokki, Cairo, 12622, Egypt
2Faculty of Science, Cairo University, Giza, Egypt
Nae-Cherng Yang NC(naeman@csmu.edu.tw)
Xiaoji Liu X(xiaoji2@illinois.edu)
Hongzhuan Xuan H(hongzhuanxuan@163.com)
Dina Mostafa Mohammed DM, The role of tamoxifen and some bioactive compounds in resistance to the development of toxicity causing breast cancer in experimental animals(2018)SDRP Journal of Food Science & Technology 3(5)
Background: Prevention or tumor resistance regards as one of the main strategies that focus on non-progression of the disease, reduction of the body's reaction to the pathogen and also one of the main factors that this research was made to study them. Methods: Thirty female Sprague-Dawley rats received a single medical dosage of 7,12-dimethylbenz[a]anthracene (DMBA) intragastrically. After fourteen days of DMBA admission, the procedure protocol started out. By the end of the experiment (6 months), apoptosis, 8-OHdG, ErbB-2, plasma Estrogen, lipid peroxidation and antioxidants were determined. Finally, all the experimental results assessed, tabulated and statistically analyzed. Results: It demonstrated a significant reduction in each of apoptosis and lipid peroxide in all prevention groups compared to injected animals' control. Alternatively, the results demonstrated an extremely significant elevation in each of 8-OHdG and Estrogen in every prevention groups. ErbB-2 results demonstrated a significant reduction in group 1 (yeast), and significant elevation in group 2 (tamoxifen). Total antioxidant results demonstrated a significant elevation in each of group 1&3 (yeast and isoflavone), and a significant reduction in group 4 (silymarin). Conclusion: Prevention may be our best tool in the battle against the disease destructive effects. Also for the tumor patient, diet can make an essential difference on the path to recovery.
Keywords: Bioactive Components, Breast Cancer, Prevention, Rats, Tamoxifen -.
LIST OF ABBREVIATIONS
DMBA, 7,12-dimethylbenz[a]anthracene; 8-OHdG, Rat 8-Hydroxy desoxyguanosine; ErbB-2, Rat receptor tyrosine-protein kinase; MDA, lipid peroxide (malonaldehyde); Bcl-2, breast cancer genes 1 and 2; ROS, reactive oxygen species; TAM, Tamoxifen; TEM, transmission electron microscope; REs, estrogen receptors.
The breast cancer etiology is multifactorial. Most of the risk factors that raise the chance of a woman creating breast malignancy have been distinguished by many epidemiologic studies which integrate early age at menarche, late age of menopause, non-reproduction, overweight, oral contraception, diet, genealogy, female sex, insufficient of physical exercise, alcohol beverages intake, hormone replacement therapy, amid menopause, ionizing radiation, early age primarily monthly cycle, older age and hereditary factors [1, 2]. There are numerous lines of breast malignancy treatments rely on the tumor stage. They include hormone-blocking therapy, chemotherapy, monoclonal antibodies, and radiotherapy [3].
Only a little minority of breast cancer patients develop the disease because of inheritance of germline mutations in prominent, highly penetrant susceptibility genes such as BRCA1 and BRCA2 [4]. At this time, additional tumor heterogeneity is placed whereby a little group of cells within, or may be external; the tumor is both secured to drugs and offers the source of new tumor progress [5]. These cells also contribute directly to the seeding of supplementary tumors at definite sites, the primary cause of mortality in breast cancer patients [6]. These drug-resistant malignancy initiating cells also known as Breast Cancer Stem Cells (BCSCs) have been exhibited functionally for both human being and mouse mammary tumors and tumor cell lines [7]. Experiments on human breast tumors in mouse models, for example, show that whenever these cells were deleted, all of those other cells weren't in a position to maintain new tumor progress [8]. So., the prevention or cancer degree of resistance of the top strategies also give attention to non-progression of the disease, limitation of the body's reaction to the pathogen also one of the main important factors that this research was designed to study them. Numerous studies demonstrated that 7,12-dimethylbenz[a]anthracene (DMBA) may be utilized to induce experimental breast carcinomas in rats and that this process includes disruption of tissue redox balance; subsequently, this demonstrates that biochemical and pathophysiological disruption may derive from oxidative destruction [9]. Under normal physiological conditions, any free radicals made in subcellular compartments would subsequently be scavenged by antioxidant defense systems of the matching cells [10]. Where, guarding systems can be destroyed easily by chemicals, such as DMBA, which disrupt the pro-oxidant-antioxidant balance, leading to cellular anomalies. The carcinogenicity of DMBA in rats was accompanied by a significant elevation in the actions of antioxidant enzymes, as a response to the induced oxidative stress and creation of reactive oxygen species [11].
Several tissues can handle activating DMBA, and these include the mammary gland. Within the breast, DMBA is altered to epoxides, effective metabolites with a convenience of destroying the DNA molecule, the key event in carcinogenesis initiation. With the higher cellular proliferative index of types 1 and 2 lobules, there may be certainly higher metabolic activity plus much more epoxide creation. Rat mammary carcinomas are started to appear in small mammary ducts [12-14] or from hyperplastic alveolar nodules [15, 16]. The breast malignancy occurrence has been elevating worldwide. Breast cancer treatments such as chemotherapy, radiotherapy, and other treatments cause serious adverse side effects. Lately, attention has been centered on identifying nutrients and food additives that have the capability to suppress the carcinogenesis processes. Therefore, it is of interest to search for new anticancer agents possessing a different mode and minimum side effects.
Tamoxifen (TAM) is the first-line endocrine treatment for premenopausal women with breast cancer and degree of resistance improvement [17, 18]. Mechanisms of resistance can conclude pharmacologic mechanisms, reduction or changes in estrogen receptor expression, modification in the regulatory proteins that be a part of several cellular operations; inhibition manipulated by the Bcl-2 family and altered mRNA expression [19]. These undesirable effects led to the utilization of different treatments of apoptosis such as complementary and alternative medicine [20]. Development and progression of breast tumors include a complex series of incidents, including dysregulation of cellular differentiation, extreme proliferation, and degree of resistance to apoptosis [21, 22]. Silymarin has been proven to possess anti-angiogenic property in several types of malignancies, which is one of the essential treatments for malignancy, by the mechanism of reducing vascular endothelial growth factor and matrix metalloproteinase-2 secretion. It had been reported that lipid peroxidation can result in MDA-DNA adduct creation, which causes frameshift mutations as a link between oxidative stress and malignancies. Isoflavones are structurally like the human female hormone 17β-estradiol; they can bind to estrogen receptors (ER) and also have estrogen-like so allow them to display estrogenic action in various tissues. Because of the structural resemblance with 17β-estradiol, isoflavones mediate almost all of their biological effects through the modulation of estrogen-receptor signaling pathways. This work is designed to study the tamoxifen drug effect on minimizing the development and cancer spread, it was also researched the consequences of some bioactive components such as yeast, Isoflavone, and silymarin to find out the various effects between them on minimizing the cancer progress to create new ways to treatment. As well, biochemical parameters which indicate lowering the growing breast cancer and limit the body's response to the toxic material through activation process of apoptotic and the forming of apoptosis thus as of 8-OHdG level plus to measure the level of ErbB-2, plasma Estrogen, as well as oxidative stress and antioxidants.
The carcinogen 7,12-dimethylbenz[a]anthracene (DMBA) with a chemical formula C20H16, molecular weight 256.35 g/mol, was purchased from Sigma Chemical Company (Sigma Chemicals, Sigma-Aldrich, St. Louis, MO, USA). The DMBA was used to induce mammary carcinoma in rats by a single dose (25 mg/kg body weight) [23].
2.1. Experimental animals:
Thirty female Sprague-Dawley rats which induced breast cancer received a single dose (25 mg/kg body weight) of 7,12-dimethylbenz[a]anthracene (DMBA) intragastrically by gavage, which was described by (Ramar et al., 2006) [23]. Two weeks after DMBA treatment, a time by which the animals had recovered from DMBA-induced toxicity, the rats were divided into 5 groups (6 for each), A group of injected animals fed on the basal synthetic diet that served as control. Injected animals (4 groups) fed on the basal synthetic diet supplemented with one source of (yeast, tamoxifen, isoflavone, and silymarin) respectively, as illustrated in table (1). The composition of salt and vitamin mixture is prepared according to the methods of (Briggs and William, 1963) [24] and (Morcos, 1976) [25] respectively.
Table (1): Composition of treatments
|
Treatments
Ingredients (g) |
Treat 1 (Yeast) |
Treat 2 (Tamoxifen) |
Treat 3 (Isoflavone)
|
Treat 4 (Silymarin) |
|
Casein Saturated/unsaturated fat Sucrose Maize starch Cellulose Salt mixture Vitamin mixture
Drugs Tamoxifen
Bioactive components Isoflavone Silymarin
Nutrient Yeast
|
15 10 22 37.333 4 4 1
---
--- ---
6.667
|
15 10 22 43.7 4 4 1
0.3
--- ---
---
|
15 10 22 43.5 4 4 1
---
0.5 ---
---
|
15 10 22 43.5 4 4 1
---
--- 0.5
--- |
|
Total |
100 |
100 |
100 |
100 |
The all groups (table 1) fed on the basal synthetic diet (basal synthetic diet is composed of casein (150 g/1 kg diet), unsaturated fat (100 g/1 kg diet), sucrose (220 g/1 kg diet), maize starch (440 g/1 kg diet), cellulose (40 g/1 kg diet), salt mixture (40 g/1 kg diet) and vitamin mixture (10 g/1 kg diet) and this diet for the control group and the other) supplemented with the normal and nano materials with different doses of each material as the following: Yeast is 6.667 g/kg diet, Tamoxifen is 3.3 mg/kg BW/day, Silymarin is 50 mg/Kg BW /day, Isoflavone is 50 mg/Kg BW /day.
2.2. Plasma and Serum Biochemistry:
At the end of experiments (six months), a blood sample of each animal collected where serum and plasma are separated by centrifugation at 3000 rpm for 15 minutes and stored in -20 0C to measure the biochemical parameters including apoptosis, 8-OHdG, ErbB-2, plasma Estrogen, as well as lipid peroxidation and antioxidants according to the following methods:
2.3 Histopathological examination of the mammary glands:
It was done according to the methods of Bancroft et al., 1996 [26]. Tissues of mammary glands from all groups were fixed in 10% buffered formalin, embedded in paraffin, sectioned into 4 μm pieces and stained with Haematoxylin-Eosin (H&E) and examined using optical microscopy.
2.4 Statistical analysis:
Data were analyzed statistically according to the method of George and William, 1989 [27]. All parameters within the given groups were evaluated through statistical software packages, namely (t-test, SPSS Software, version 16.0 for Windows; SPSS Inc., Chicago, IL).
3.1 Results:
Histopathological examination of the mammary glands:
1- Control (injected animals) (Fig. 1 a&b):
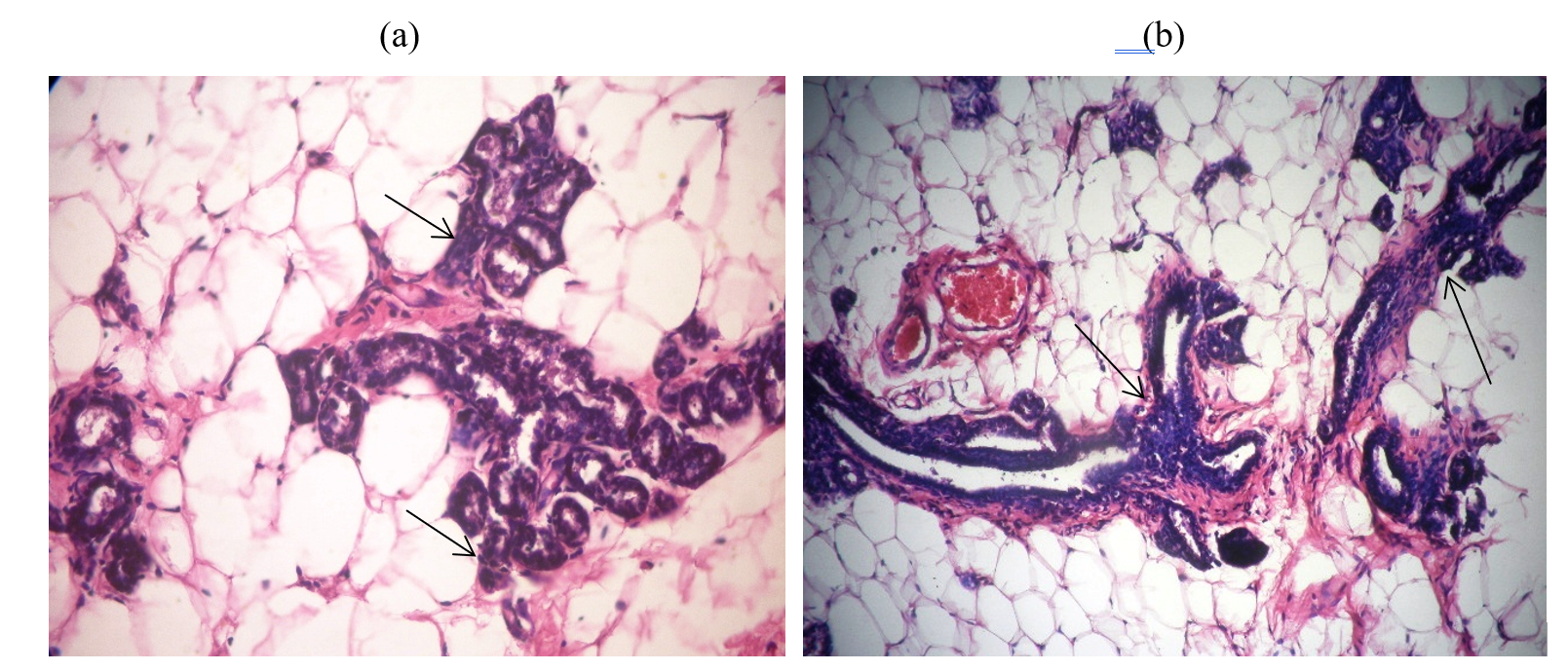
Fig. 1.a: Mammary gland of rat showing well-differentiated adenocarcinoma (H&E ×400).
Fig. 1.b.: Mammary gland of rat showing ductal hyperplasia (H&E ×400).
2- Normal Control (Fig. 2 a&b):
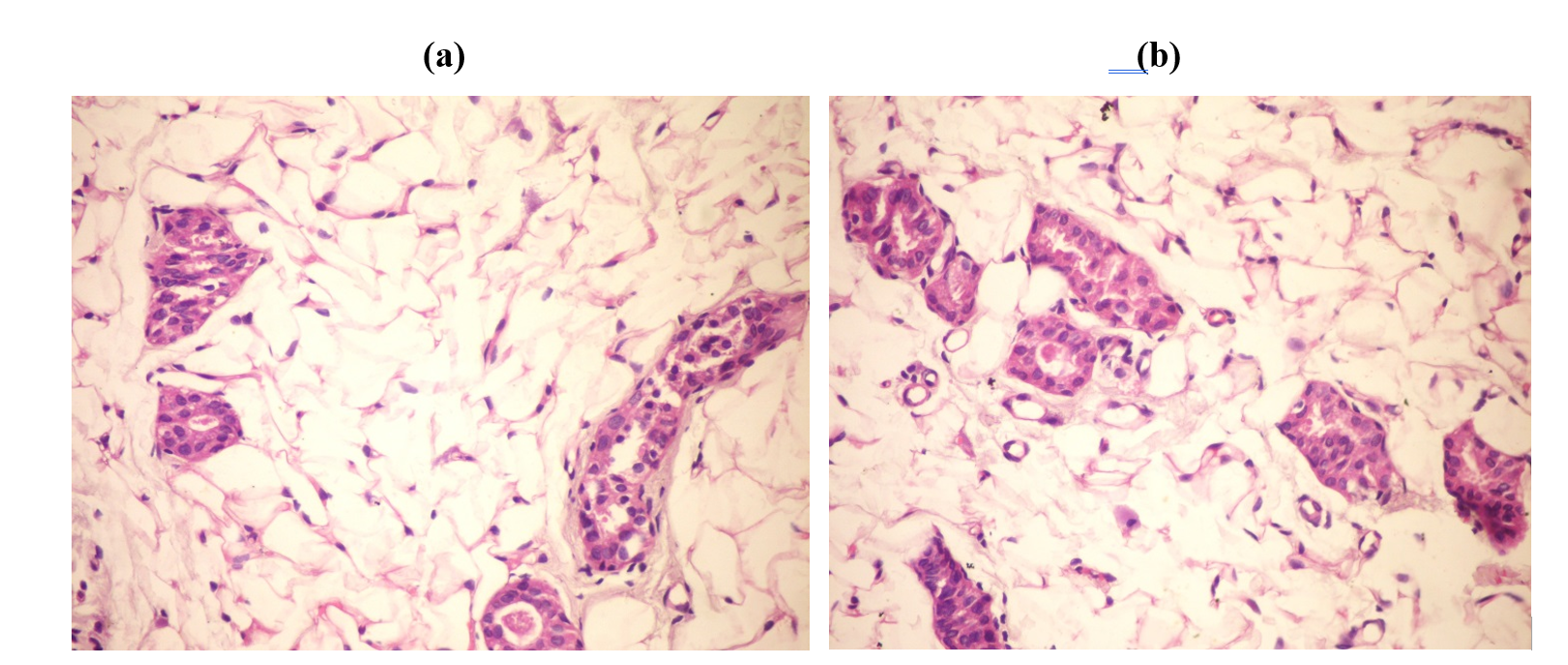
Fig. 2.a.: Mammary gland of rat showing no histopathological changes (H&E ×400).
Fig. 2.b.: Mammary gland of rat showing normal mammary acini (H&E ×400).
Table (2): The different effects of tamoxifen and bioactive components on apoptosis, 8-OHdG, ErbB-2, Plasma Estrogen, Lipid peroxide and Total antioxidant.
|
Group |
Apoptosis Pg/ml |
8-OHdG ng/L |
ErbB-2 pg/ml |
Plasma Estrogen pg/ml |
Lipid peroxide nmol/ml |
Total Antioxidant mM/L |
|
Control (injected) (+ve)
Mean ± SE |
35.17 ± 1.492 |
4.35 ± 0.382 |
691.3 ± 47.43 |
99.6 ± 2.28 |
29.63 ± 1.89 |
0.795 ± 0.01 |
|
Group (5) (Normal Yeast) Mean ± SE |
19 ± 3.3*** |
5.7 ± 0.14*** |
578.7 ± 20.6** |
126.5 ± 1.73**** |
8.9 ± 0.22**** |
0.85 ± 0.014*** |
|
Group (6) (Normal Tamoxifen) Mean ± SE |
26.0 ± 2.8** |
6.0 ± 0.15*** |
1593 ± 130.8**** |
123 ± 1.89**** |
11.4 ± 0.59**** |
0.77 ± 0.02N.S |
|
Group (7) (Normal Isoflavone) Mean ± SE |
25.2 ± 3.11** |
6.7 ± 0.02**** |
626 ± 16.15N.S |
126.7 ± 3.76**** |
17.4 ± 0.48**** |
0.73 ± 0.011*** |
|
Group (8) (Normal Silymarin) Mean ± SE |
20.8 ± 1.17**** |
6.1 ± 0.03**** |
849.5 ± 63.8N.S |
136.8 ± 1.25**** |
14.8 ± 1.28**** |
0.84 ± 0.014*** |
It was noticed a significant reduction in each of apoptosis and lipid peroxide in all prevention normal groups compared to injected animals' control. Alternatively, the results demonstrated an extreme significant elevation in each of 8-OHdG and Estrogen in all prevention groups compared to injected control. ErbB-2 results demonstrated a significant reduction in group 1 (yeast), and a significant elevation in group 2 (tamoxifen). The results of total antioxidant demonstrated a significant elevation in each of group 1&3 (yeast and isoflavone). And a significant reduction in group 4 (silymarin) compared to injected animals' control.
Breast carcinoma is regarded as one of the very most well-known neoplasms in women as well as the reason behind cancer-related deaths. Consequently, the prevention or cancer degree of resistance is of the main strategies also center on non-progression of the disease, minimizing of the body's response to the pathogen also one of the main factors that this research was created to study them. Many studies had demonstrated that 7,12-dimethylbenz[a]anthracene (DMBA) may be utilized to induce experimental breast carcinomas in rats and this process concludes dysfunction of tissue redox balance; consequently, this demonstrates that biochemical and pathophysiological disorders may derive from oxidative damage [9]
The results demonstrated anatomical injury to animals in breast cancer because of injection of DMBA triggering breast cancer. It had been already noticed results by injection DMBA events breast tumor through microscopic examination of breast cells infected rats compared to normal animals were also observed from the present study results (Fig. A and B).The results were agreed with many authors which reported that (DMBA)-induced mammary tumors in the Sprague–Dawley rat model.
The results demonstrated a significant reduction in the apoptosis level in all the groups compared to control animal disease (table 2); the reduction in apoptosis level was associated with a significant reduction in the lipid peroxidation level. However, the results demonstrated a high 8-OHdG level in all groups of animal disease compared to control. In the same line, the results also demonstrated a significant elevation in ErbB-2 level was noticed in the tamoxifen group. The present work expands our knowledge of how tamoxifen kills breast cancer cells and it elucidates the adaptive mechanisms that reduce the efficacy of tamoxifen treatment. The concentrations of tamoxifen and its own metabolites arise in breast tumors of patients [29]. A major part of this effect was through oxidative stress triggered by tamoxifen accumulation since the malignant cells were partially shielded from apoptosis by the antioxidants. Tamoxifen partitions into lipid membranes leading to increased oxidative destruction [30]. Tamoxifen treatment increased the superoxides formation and the lipid peroxidation in breast malignancy cells. Other studies demonstrated that adding tamoxifen increased the amount of the marker for oxidative stress (8-OHdG) on the DNA. This oxidative destruction on DNA was diminished by enzymes of antioxidant.
Conversely, the significant elevation in total antioxidants in the silymarin group didn't lead to a big change in ErbB-2 level in that group as happened in the yeast group. From numerous reports, it proved that silymarin has cytoprotection activities because of its antioxidant activity and radical scavenging. Antioxidants are regards as defensive agents that inactivate ROS and play an essential role in the cells protection from oxidative destruction. It's obvious that patients with breast cancer suffer from chronic oxidative stress and also have an altered redox state characterized by gross depletion of antioxidant nutrients [31, 32]. Clinical occurrences in breast tumor patients take part indirectly by severe antioxidant depletion leading to inadequate protection. Therefore, specified medical trials of the utilization of antioxidants sources may create useful information to regulate oxidative stress. Constituents in foods may be a tumor causing, cancer-promoting or defensive against cancer [33]. Currently, prevention may be our best tool in the battle against the destructive effects of the disease. Also for the cancer patient, diet can create a crucial difference on the path to recovery [33]. It had been reported that the mean lipid peroxide levels in the control group were minimized in people that have benign breast disease. Furthermore, the mean serum lipid peroxides levels were very low, cases with proven breast cancer diagnosis. Regardless of the restrictions in the study, it was found lower serum lipid peroxide levels in breast cancer patients, a finding that is supported by prior research [34]. So., many enzymes in the body, also it works as a direct scavenger of free radicals and protects from oxidative destruction. But in the case of the imbalance between oxidant and antioxidant, the defense mechanism manages to lose the battle. Numerous enzymes are rendered functionless and can result in the sequestration of microelements in the bloodstream. Several studies conducted in recent times demonstrate that increased oxidative stress and lipid peroxidation is implicated in its carcinogenesis [35] Also, studies demonstrate a positive linkage between lipid peroxidation and malignancy [36]. The many circulating enzymatic and non-enzymatic antioxidants were minimized in several women with breast cancer [37]. The above-mentioned studies results are relative to that presented in another research which demonstrates a minimal level of antioxidant status in the women with breast cancer. Numerous studies which demonstrated a rise in a number of antioxidant enzymes recommended that the ROS generated might stimulate the antioxidant enzymes to be able to get rid of the extreme free radicals which were produced. These studies agreed with the present study in groups and differed with other groups. However, the body’s defense mechanisms play a key role by means of antioxidants that help to minimize the damages which are triggered by oxidative stress. Antioxidants are substances that get rid of, scavenge and inhibit the free radicals formation or oppose their activities. The results demonstrated a significant reduction in ErbB-2 level in the yeast group. Conversely, a significant elevation in ErbB-2 level was noticed in the tamoxifen group. Possibly the indicator of elevating total antioxidant level in the yeast group caused a significant reduction in ErbB-2 level. It had been reported that ErbB-2 (HER-2) is often overexpressed in breast cancer.
The experiment of prevention to disease by nourishing on bioactive components demonstrated a significant elevation in the estrogen level in all groups compared to control. There is certainly adequate experimental, epidemiological, and medical proof linking estrogens and breast cancer risk [38]. Several proven risk factors for breast malignancy are firmly linked with sex hormone levels, recommending these factors affect estrogen signaling pathways to impact breast cancer risk [39].
The results demonstrated a notable difference in the total antioxidant level in the groups, in which a significant elevation was noticed in yeast and silymarin. Numerous reports have regarded that silymarin is regarded as an essential way to obtain antioxidants. From many reports, it was proved that Silymarin has cytoprotection activities because of its antioxidant activity and radical scavenging. The possible known mechanisms of action of silymarin protection are blockade and modification of cell transporters, β-glycoprotein, estrogenic and nuclear receptors. Antioxidant properties have been reported for silymarin, which elevates the antioxidant enzymes activity [40]. Effects of silymarin on breast cancer have been reported. Mechanism of cytoprotective activity of silymarin belongs to antioxidative and radical scavenging results as well as the precise receptor interaction and modulation of a number of cell-signaling pathways. The multidrug level of resistance is one of the key problems of successful cancer treatment, silymarin elevates absorption and bioavailability of chemo Pharmaceutics in cancerous cells by suppression of β -glycoprotein-mediated drug carrier and breast cancer resistance healthy protein. It's been demonstrated that silymarin has the growth inhibitory effect of cell proliferation suppression and apoptosis induction. Therefore, the present study results were agreed with several reports and differed with some. A higher level of antioxidants in prevention yeast group may be linked with the yeast synthesis as a way to obtain vitamin B complex and the activation of natural antioxidant enzymes by the body which resulted in a high antioxidant level in the yeast group compared to the control.
The isoflavones group demonstrated a significant reduction in the total antioxidant level, even though it consists of a number of antioxidants and phytochemical substances. It had been reported that several mechanisms have been proposed for the biological activity of isoflavones since they can work as antioxidants, and free radical-scavengers, and can protect cells against ultraviolet (UV)-induced destruction. Perhaps those mechanisms resulted in a minimal level of antioxidants so they can modify the higher level of free radicals. Studies have verified increased oxidative stress because of illness, which includes breast cancer. It had been reported that historically low breast cancer occurrence and mortality rates in soy food-consuming countries helped fuel the initial desire for this relationship as performed the identification of isoflavones as possible chemopreventive agents. These diphenolic substances, which are found in uniquely-rich quantities in soybeans, have got both estrogen-dependent and -independent properties that probably allow these to inhibit the breast cancer progression. Furthermore, substantial specialized medical research implies the estrogen-like properties of isoflavones may be especially good for peri- and postmenopausal women. For instance, isoflavones relieve hot flashes, may progress arterial health, minimize wrinkles and elevate the prognosis of breast cancer patients. Isoflavones have an identical chemical composition to estrogen, bind to estrogen receptors (ERs), and exert estrogen-like effects under certain experimental conditions. For these reasons, they are categorized as phytoestrogens. Proof implies that ERβ functions as a tissue-specific tumor suppressor with antiproliferative activities. There are many clinical types of isoflavones exerting estrogen-like effects in some tissues but having no influence on other estrogen-sensitive endpoints, although there is bound proof demonstrating anti-estrogenic effects. Finally, various mechanisms for the protective effects of early isoflavone exposure have been suggested. It would appear that isoflavones change cells in the growing breast with techniques that produce them permanently less likely to be altered in cancer cells. Isoflavones are structurally like the human female hormone 17β-estradiol; they can bind to estrogen receptors (ER) and also have estrogen-like so allow them to display estrogenic action in various tissues. Because of the structural resemblance with 17β-estradiol, isoflavones mediate almost all of their biological effects through the modulation of estrogen-receptor signaling pathways. In hormone-dependent tissues, estrogens play an effective role in various physiological processes, such as cell proliferation, differentiation or apoptosis. However, high estrogen levels are the main risk factor for the progression of hormone-dependent diseases, such as breast cancer. It is still not completely clear why endogenous or artificial estrogens elevate breast cancer risk, while phytoestrogens, structurally similar substances, may actually have the contrary impact. Numerous in vitro studies demonstrate that isoflavones inhibit cell proliferation and cause apoptosis by inhibiting the action of several enzymes, such as tyrosine protein kinase, Furthermore to these, isoflavones, enhance antioxidant defense and DNA repair, inhibit the progression of tumor angiogenesis and metastasis. Additionally, isoflavones can initiate apoptotic events, suppress angiogenesis signaling pathways or interfere in the redox state of the cells. Everything considered, there continues to be a keen desire for discovering isoflavones chemopreventive properties as cellular mechanisms aren't fully realized. Estrogen is thought to be involved in breast malignancy improvement and progression, and any dietary treatment that blocks the creation or minimizes the hormone action is likely to be effective in progressing clinical final results in breast tumor survivors.
Tamoxifen partitions into lipid membranes resulting in increased oxidative damage. It also elevated the level of oxidative stress (8-OHdG) on the DNA. Silymarin has cytoprotection activities due to its antioxidant activity and radical scavenging. Perhaps the indicator of the elevating level of total antioxidants in the yeast group led to a significant reduction in ErbB-2 level. Yeast is regarded as a vitamin B complex source and the activation of natural antioxidant enzymes by the body resulted in an extreme level of antioxidants in the yeast group. Isoflavones can initiate apoptotic occurrence, suppress angiogenesis signaling pathways or interfere in the redox state of the cells. Everything considered, there continues to be a keen desire for discovering isoflavones chemopreventive properties as cellular mechanisms aren't fully realized. Prevention may be our best tool in the battle against the disease destructive effects. Also for the tumor patient, diet can make an essential difference on the path to recovery.
I want to thank all the authors for this study and also National Research Centre.
Brody JG, Rudel RA, Michels KB, et al.: Environmental pollutants, diet, physical activity, body size, and breast cancer: where do we stand in research to identify opportunities for prevention?. Cancer 2007; 109: 2627-2634. PMid:17503444
View Article PubMed/NCBIZhang Y, Lv F, Yang Y, et al.: Clinicopathological Features and Prognosis of Metaplastic Breast Carcinoma: Experience of a Major Chinese Cancer Center. PLoS One 2015; 10: e0131409. PMid:26115045 PMCid:PMC4482719
View Article PubMed/NCBISantana-Davila R and Perez EA: Treatment options for patients with triple negative breast cancer. J Hematol Oncol. 2010; 3: 42. PMid:20979652 PMCid:PMC2987865
View Article PubMed/NCBIBadve S and Nakshatri H: Oestrogen-receptor-positive breast cancer: towards bridging histopathological and molecular classifications. J Clin Pathol 2009; 62:6-12. PMid:18794199
View Article PubMed/NCBIZhou BB, Zhang H, Damelin M, et al.: Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009; 8:806-823. PMid:19794444
View Article PubMed/NCBICharafe JE, Ginestier C, Iovino F, et al.: Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res 2009; 69:1302-1313. PMid:19190339 PMCid:PMC2819227
View Article PubMed/NCBIAl-Hajj MB, Wicha MW, M Weissman I, et al.: Therapeutic implications of cancer stem cells. CurrOpin Genet Dev. 2004; 14:43-47. PMid:15108804
View Article PubMed/NCBILuke PN, Omidvar SM., Pérez ME, et al.: Suppression of apoptosis inhibitor c-FLIP selectively eliminate breast cancer stem cell activity in response to the anti-cancer agent, TRAIL. Breast Cancer Research 2011; 13: R88. PMid:21914219 PMCid:PMC3262200
View Article PubMed/NCBIHenry L and Narendra PS: Oral artemisinin prevents and delays the development of 7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the rat. Cancer Lett 2006; 231:43–4. PMid:16356830
View Article PubMed/NCBISalet C and Moreno G: Photosensitization of mitochondria, Molecular, and cellular aspects. J Photochem Photobiol, B 1990; 5:133–50. 80002-F
View ArticleFang J, Seki T and Maeda H: Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev 2009; 61:290–302. PMid:19249331
View Article PubMed/NCBIMiddleton PJ: The histogenesis of mammary tumors induced in the rat by chemical carcinogens. Br J Cancer; 19(4) (1965):830–839. PMid:4285918 PMCid:PMC2071390
View Article PubMed/NCBISinha D and Dao TL: Site of origin of mammary tumors induced by 7,12-dimethylbenz[a]anthracene in the rat. J Natl Cancer Inst. 1975; 54(4):1007–1009. PMid:805252
PubMed/NCBIRusso J, Saby J, Isenberg WM, et al.: Pathogenesis of mammary carcinomas induced in rats by 7, 12-dimethylbenz[α]anthracene. J Natl Cancer Inst. 1977; 59:435–445.
View ArticleBeuving LJ: Effects of Ovariectomy on Preneoplastic Nodule Formation and Maintenance in the Mammary Glands of Carcinogen-Treated Rats. J Natl Cancer Inst. 1969; 43(5): 1181-1189. PMid:5353244
PubMed/NCBIBeuving LJ and Bern HA: in Estrogen Target Tissues and Neoplasia, ed. Dao, T. L. (University of Chicago Press, Chicago, IL), 1972, pp. 257-273.
Brown K: Is tamoxifen a genotoxic carcinogen in women?. Mutagenesis 2009; 24: 391–404. PMid:19505894
View Article PubMed/NCBIJiang Q, Zheng S, Wang G: Development of new estrogen receptor targeting therapeutic agents for tamoxifen-resistant breast cancer. Future Med Chem. 2013; 5: 1023-1035. PMid:23734685 PMCid:PMC3855007
View Article PubMed/NCBIViedma RR., Baiza GL, Salamanca GF, et al.: Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (Review). Oncology Reports 2014; 32: 3-15. PMid:24841429
View Article PubMed/NCBIDigianni LM, Garber JE, Winer EP: Complementary and alternative medicine use among women with breast cancer. J Clin Oncol 2002; 20: 34S-38S. PMid:12235222
PubMed/NCBIHanahan D and Weinberg RA: Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. PMid:21376230
View Article PubMed/NCBIKumaraguruparan R, Balachandran C, Manohar BM, et al.: Altered oxidant-antioxidant profile in canine breast tumors. Vet Res Commun 2005; 29:287–96. PMid:15751580
View Article PubMed/NCBIRamar PS, Ponnampalam G, Savarimuthu I: Anti-tumor promoting the potential of luteolin against 7,12-dimethylbenz[a]anthracene-induced mammary tumors in rats. Chemico-Biological Interactions 2006; 164:1–14. PMid:17064676
View Article PubMed/NCBIBriggs GM and William MA: A new mineral mixture for experimental rat Diets & evaluation of other mineral mixture Fed proc. 1963, 122: 261-266.
Morcos SR: The effect of protein value of the diet on the neurological manifestations produced in Rats by β-immodipropionitrite. Br. J. Nutr. 1976; 21: 269-274.
View ArticleBancroft D and Stevens A: Theory & practice of histological techniques. Fourth edition. Churchill Livingstone, Edinburgh, London, Melbourne,1996.
Snedecor GW and Cochron WG: Statistical Methods, 8th ed., Lowa State Unill. Press, Ames, Lowa, USA, 1989.
Black K: Business statistics for contemporary decision making; copyright 2004; Leyh Publishing, LLC. 2004.
Kisanga ER, Gjerde J, Guerrieri GA, et al.: Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res 2004; 10: 2336–2343. PMid:15073109
View Article PubMed/NCBIGundimeda U, Chen ZH and Gopalakrishna R: Tamoxifen modulates protein kinase C via oxidative stress in estrogen receptor-negative breast cancer cells. J Biol Chem. 1996; 271: 13504–13514. PMid:8662863
View Article PubMed/NCBIKulkarni SD, Tilak JC, Acharya R, et al.: Evaluation of the antioxidant activity of wheat grass (Triticum aestivum L.) as a function of growth under different conditions. Phytother Res. 2006; 20:218-27. PMid:16521113
View Article PubMed/NCBIBar-Sela G, Tsailic M, Fried G, et al.: Wheat grass juice may improve hematological toxicity related to chemotherapy in breast cancer patients: a pilot study. Nutr Cancer 2007; 58(1):43-8. PMid:17571966
View Article PubMed/NCBIWhitney EN, Hamilton EMN, Rolfes SR: Understanding Nutrition West Publishing Company 5th Edition St. Paul, 1990, pp. 514-519.
González MJ, Schemmel RA, Dugan L Jr., et al.: Dietary fish oil inhibition of human breast carcinoma growth: a function of increased lipid peroxidation. Lipids 1993; 28: 827-832. PMid:8231658
View Article PubMed/NCBIKhanzode SS, Muddeshwar M, Khanzode SD, et al.: Antioxidant enzymes and lipid peroxidation in different stages of breast cancer. Free Radic Res. 2004; 38:81-5. PMid:15061657
View Article PubMed/NCBIErten Sener D, Gonenc A, Akinci M, et al.: Lipid peroxidation and total antioxidant status in patients with breast cancer. Cell Biochem Funct 2007; 25:377-82. PMid:16447143
View Article PubMed/NCBIYuvaraj S, Premkumar VG, Vijayasarathy K, et al.: Augmented antioxidant status in Tamoxifen treated postmenopausal women with breast cancer on co-administration with Coenzyme Q10, Niacin and Riboflavin. Cancer chemotherapy and pharmacology 2008; 61(6): 933-941. PMid:17668211
View Article PubMed/NCBIRusso J and Russo IH: The role of estrogen in the initiation of breast cancer. Journal of Steroid Biochemistry and Molecular Biology 2006; 102: 89–96. PMid:17113977 PMCid:PMC1832080
View Article PubMed/NCBIKey TJ, Appleby PN, Reeves GK, et al.: Circulating sex hormones and breast cancer risk factors in postmenopausal women: reanalysis of 13 studies. British Journal of Cancer 2011; 105: 709–722. PMid:21772329 PMCid:PMC3188939
View Article PubMed/NCBIGholamreza K, Maryam V, Parisa L, et al.: Silymarin", a Promising Pharmacological Agent for Treatment of Diseases. Iranian Journal of Basic Medical Sciences 2011; 14(4): 308-317.
