Karen Ovsejevi Gandara
Email: kovsejev@fq.edu.uy ; Telephone: + 598 2 9241806; Fax: + 598 2 9241906
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 2
Page No: 269-279
Karen Ovsejevi Gandara
Email: kovsejev@fq.edu.uy ; Telephone: + 598 2 9241806; Fax: + 598 2 9241906
Gabriela Peralta-Altier, Carmen Manta, Karen Ovsejevi*
Cátedra de Bioquímica, Departamento de Biociencias, Facultad de Química, Universidad de la República, General Flores 2124, Montevideo, Uruguay
Tri M Bui-Nguyen(Tri.Bui-Nguyen@fda.hhs.gov)
Umberto Mura(umberto.mura@unipi.it)
Halil Ozkol(h_ozkol@mynet.com)
Karen Ovsejevi, Thiol-Cyclodextrin: A New Agent For Controlling The Catalytic Activity Of Polyphenol Oxidase From Red Delicious Apple(2018)SDRP Journal of Food Science & Technology 3(2)
Polyphenol oxidase (PPO, EC 1.14.18.1) is the main enzyme responsible for enzymatic browning, a natural process which produces deterioration of fruits and vegetables. One alternative to prevent this undesirable process is to inhibit the catalytic activity of PPO by encapsulating enzyme’s substrates in cyclodextrins (CD). In this article the effect of a Thiol-CD on PPO from Red Delicious apple was studied, demonstrating that this compound is a powerful tool for controlling oxidative processes in food. Thiol-CD could encapsulate the polyphenols, natural substrates of the enzyme, by means of the cyclodextrin hydrophobic internal cavity. Simultaneously, through the thiol group, it could inactivate the PPO by reducing the copper ions from the active site of the enzyme. Moreover, thiol moieties could decrease the browning by reducing the quinones generated by oxidative processes. The isolation and purification of PPO from apple was performed in order to study those effects, different polyphenols, chlorogenic acid (CA) and 4-methylcatechol (4-MC), were assayed as enzymatic substrates. Both β-CD and Thiol-CD exhibited better enzyme inhibition value for CA than for 4-MC. Moreover, Thiol-CD showed an extraordinary performance compared with β-CD. When CA 10 mM was used, 5 mM β-CD gave 11 % PPO inhibition, meanwhile nearly 100 times less concentration of Thiol-CD (45 μM) gave 100 % of enzyme inhibition.
Keywords
Antibrowning agents, enzymatic browning, oxidative processes in food.
The demand for sliced fresh fruit and vegetables has constantly increased during the last decade, due to ongoing lifestyle choices combined with the necessity of having a healthy diet. However, enzymatic browning is one of the most important drawbacks for introducing fresh fruits and vegetables with minimal processing in the market. This enzymatic process lowers fruit quality, changing aspect, taste and nutritional characteristics [1].The major enzyme responsible for this damage is polyphenol oxidase (PPO), a metalloprotein with two Cu (II) in its active site, which are essential for its activity. In the presence of oxygen PPO oxidizes o-diphenols to quinones and leads to brown pigments (Figure 1) [2-5].
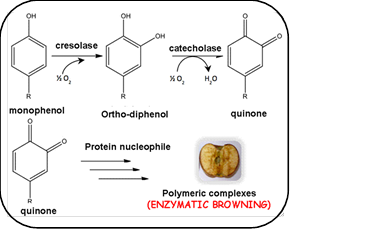
Figure 1- Mechanism of enzymatic browning
Inhibition of enzymatic browning can proceed through different mechanisms: direct inhibition of the enzyme, non-enzymatic reduction of o-quinones (which are formed by enzymatic oxidation of o-phenols) or by removal of phenolic substrates of PPO [2].
Cyclodextrins (CDs) have been used as antibrowning agents mainly by encapsulation of phenols in their hydrophobic internal cavity [6-8]. CDs, cyclic oligomers composed by 6, 7 and 8 glucose units (a-, b- and g-CD respectively) have a truncated cone shape with an external hydrophilic surface and an apolar cavity. This particular structure makes CDs excellent agents for encapsulating hydrophobic substances, allowing changes in the physicochemical properties of the host molecule. Furthermore, they are neutral in terms of odor and taste; in spite of their glucose composition a- and b-CD do not taste sweet at all, while g-CD has only a slightly sweet taste, additionally they occur as a colorless powder. Moreover they are non-toxic, and they are included in the GRAS list (FDA list of food additives that are generally recognized as safe) [9]. Because of all these characteristics, they are widely used in the food industry.
Our group has successfully synthesized a Thiol-CD by an easy and eco-friendly method, giving a thiolated cyclodextrin with an average value of one thiol group per molecule (Figure 2) [10].
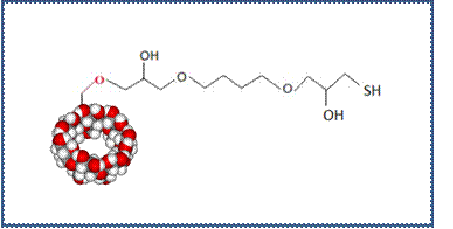
Figure. 2: Thiol-CD structure
The modified CD retained the capacity for encapsulating hydrophobic molecules, like β-CD, and additionally, behaved as a reducing agent. These properties make Thiol-CD a very promising tool for controlling PPO activity, mainly due to the encapsulation of its hydrophobic substrates and the concomitant generation of a reducing microenvironment. As a result of this, enzymatic browning, the oxidative process mediated by PPO, would be minimized.
In this work the control of enzymatic browning focused primarily on regulating PPO activity. Therefore, extraction and purification of the enzyme were essential steps to elucidate the anti-browning mechanisms of b-CD and Thiol-CD. Moreover, the significance of the PPO substrates´ structure on the encapsulation process, was analyzed by using two polyphenols with different chemical structure (Chlorogenic Acid (CA) and 4-Methylcatechol (4-MC))[11]. Evaluation of the antibrowning effect of b-CD and Thiol-CD was carried out on PPO extract and onto fresh apple.
Materials
Apples (Red Delicious) were purchased from a local green store. 4-Methylpyrocatechol (4-MC) was obtained from (MERCK, NJ, USA). b-Cyclodextrin (b-CD); Chlorogenic Acid (CA); Triton X-100; Polyvinylpolypyrrolidone (PVPP) and 1,4-Butanediol diglycidyl ether (DGE) were purchased from SIGMA (St Louis, MO, USA). Thiopropyl agarose was synthesized in our lab as reported by Batista 1996 [12]. Biotech Dialysis Membranes, Spectra/PorÒ 12000-14000 MWCO were obtained from SpectrumÒ Laboratories (CA, USA). DEAE-Sephadex A-50, Phenyl Sepharose, Sepharose 4B, PD-10 columns (Sephadex G-25), PhastGel Gradient 8-25, PhastGel IEF pH 3-9, standard proteins for sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and IEF were purchased from GE Healthcare Bio-Sciences AB (Uppsala, Sweden).
Methods
Enzymatic activity assay
PPO activity was assayed by mixing 0.9 mL of 10 mM substrate in activity buffer (0.1 M sodium acetate pH 4.6) with 0.1 mL of enzyme sample. The change in absorbance at 420 nm per 1 min was determined. CA and 4-MC were used as substrates [13]. A blank was done using the same mixture, with substrate and substituting the enzyme for activity buffer.
EU definition: One enzyme unit is the amount of enzyme necessary to produce a change of 0.001 in absorbance at 420 nm per minute at 22 °C and pH 4.6.
Protein determination
Protein content was determined using the BCA assay with bovine serum albumin as standard [14].
PPO extraction from Red Delicious apple [4, 15-18]
Organic Apples (Red Delicious) were purchased from a green store with the purpose of avoiding the mixture of chemical substances present in conventional grown apples. In order to optimize the extraction process different conditions were assessed involving changes in: Triton X-100 concentration, pH, ionic strength and buffer composition. Apple samples (25 g), and the optimized extraction buffer (40 mL, in presence of 2 % PVPP) and 100 µL of Triton X-100 were blended for 3 min using a homogenizer and stored for 1.5 h at 4 ºC. Then, the homogenate was filtered and centrifuged in order to remove the insoluble reagent PVPP and tissue debris.
Purification of Apple PPO from Red Delicious apple
Crude extract was sequentially purified by dialysis with molecular porous membrane (MWCO 12000-14000), ionic exchange on DEAE-sephadex (batchwise, in with 0.1 M sodium phosphate pH 7.4 at 4 ºC, elution in column with 0.2 M sodium phosphate pH 7.4) and hydrophobic chromatography on phenyl Sepharose with 10 mM sodium phosphate pH 7.4 , 1 M ammonium sulphate ( elution was performed with the same buffer without ammonium sulphate)
SDS-PAGE electrophoresis and isoelectric focusing (IEF) were performed on PhastSystem electrophoresis make with gradient gels 8-25 and Phastgel IEF 3-9 respectively.
Characterization of purified PPO from Red Delicious apple
PPO activity was assayed as reported above with CA and 4-MC, over a wide range of temperatures (5 to 60 °C) and pHs (3.0-7.5) to determine optimal conditions. Thermal and pH stability were assessed by incubating aliquots of enzyme (1400 UE/mL) at different conditions. For thermal assays, the incubation was run at pH 4.6 for 24 h at temperatures between 4 and 50 ºC. After the incubation period the samples were brought to room temperature and residual activity was quantified as already described. The pH stability was performed at 4 ºC for 24 h at pHs ranging from 3.0 to 8.7. Afterwards, samples pH was adjusted to 4.6 and residual activity was measured. Shelf stability was also assayed during extended periods at 4°C and pH 4.6, measuring residual activity at room temperature. The 100% of stability was the initial activity of the enzyme, without any treatment.
Kinetic parameters were also determined for both substrates (CA and 4-MC), at room temperature (22ºC) and pH 4.6, using a concentration range of 0.5 to 10 mM. Michaelis-Menten kinetics was adequate to fit the data and KM and Vmax were obtained by Lineweaver-Burk, Hannes and Eaddie –Hofstee plots.
Synthesis of Thiol-cyclodextrin
As reported, the method comprises three steps: epoxy activation of β-CD with DGE, conversion of the introduced oxyrane groups into thiosulphate groups, and their reduction to thiol groups with a solid phase reducing agent (Thiopropyl agarose).
Effect of b-cyclodextrin and Thiol-cyclodextrin on PPO activity
Both 4-MC and CA were used as substrates. The activity assay was performed following the protocol described above using different substrate´s concentrations (for CA 2.0-10.0 mM and for 4-MC 1.0-5.0 mM) and in the presence of different concentrations of β-CD (1.0 to 5.0 mM) and Thiol-CD (2.5 - 45.0 μM).
Percentage of inhibition was calculated as follow:
I (%)= 100 – (AR/ AC x 100)
AR = Residual PPO activity after antibrowning agent addition
Ac= Control PPO activity (sample without antibrowning agent)
Shelf stability of sliced apple
Organic apples (Red delicious) were cored, peeled, sliced and kept on ice. Aliquots were taken and distributed in Petri boxes. The protocol involved the soaking of 2 apple slices of 3 mm thickness with antibrowning agents, 1.0 ml of β-CD or Thiol-CD (different concentrations, in 0.1 M acetate buffer, pH 4.6). After 5 min the solution was removed and the box was closed in order to reduce the oxygen concentration. Slices with no treatment and only treated with acetate buffer were run as controls. Apple pulp color was measured at different times, from 1 to 24 h using a iWAVE WF30 Portable Accurate Colorimeter 4 mm Caliber CIELAB CIELCH Color Difference, CIE/10° was the illuminant/viewing geometry. Therefore the illuminant and observer conditions were: CIE standard illuminants with a focus angle of 10°. Three independent measurements for each of the three areas (slice extremes and middle) were monitored. Readings were expressed as L*, a* and b* parameters.
Statistical analysis
All determinations were replicated at least three times and analysis of variance (ANOVA) of the data was carried out. The Tukey test was employed to determine the statistical significance of differences between the means (p<0.05).
In order to study the simultaneous effect of β-CD and substrate concentration on PPO activity, a two way ANOVA was performed using Minitab 17 statistical software.
Extraction of PPO from Red Delicious apple
PPO in fruits and vegetables can be found free in the cytosol or associated with the thylakoid membrane of chloroplasts [19]. In order to remove PPO from membranes the enzyme extraction process from Red Delicious apple needed to be optimized. These studies were carried out in aqueous medium, using PVPP for reducing endogenous polyphenol concentration [20]. The effect of pH, ionic strength, buffer composition and Triton X-100 influence was evaluated (Figure 3).
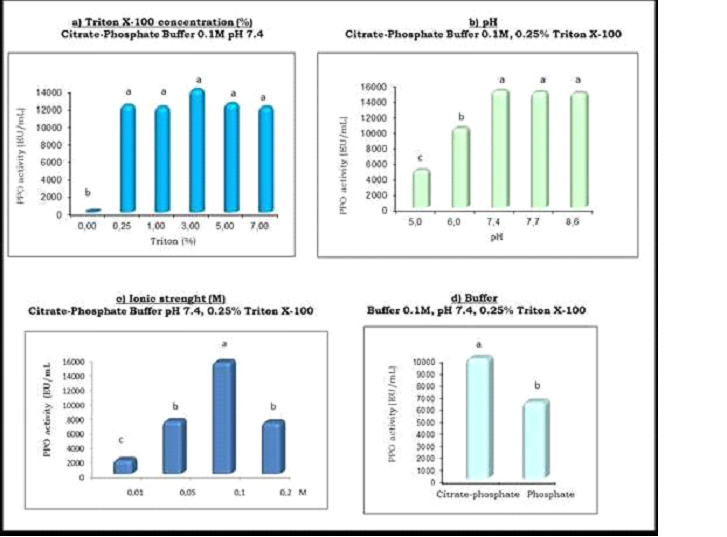
Figure 3- Optimization of PPO Extraction
Results represent averages of at least three experiments. The same letter indicates no significant difference at the p≤ 0.05 level according to Tukey’s test.
The addition of different amounts of Triton (from 0.25 to 7%) proved to be essential for membrane solubilization and therefore for enzyme recovery. However the smallest amount of reagent assayed was enough, avoiding the problematical elimination of a big excess of detergent.
It was observed that the pH of the extraction solution significantly affected the activity of the extract. The maximum activity was achieved when extraction was performed at pHs greater than or equal to 7.4. The extracts obtained at pHs 5.0 and 6.0 were less active than the one prepared at pH 7.4, with a total activity of 32% and 68% respectively.
One possible explanation for this effect would be the acidic nature of PPO, therefore as pH decreases the reaction of the enzyme with natural polyphenols (liberated during the extraction process) would be potentiated, increasing oxidation products concentration. Since these products have an inhibitory effect on PPO, the resulting extract after 24 h at acidic pHs would be less active [21].
Ionic strength was also important since the recovered activity strongly depended on buffer concentration. This could be explained because ionic strength lower than 0.1 M (optimum concentration) is not enough to neutralize the natural acidity of apple extract, compromising the stability of the enzyme. On the other hand a 0.2 M solution diminished enzyme activity, possibly due to a structural change in the active site of the enzyme because of ionic interactions.
The effect of buffer composition on PPO extraction was also studied and the highest PPO activity was achieved with citrate-phosphate buffer. This buffer could behave different from phosphate buffer, generating ion–protein interactions that would allow an additional stabilization of enzyme´s structure.
The extraction process was also strongly dependent on the selected physical method the best results were achieved using a mixer instead of a magnetic stirrer.
In summary, an apple PPO extract with high enzymatic activity (16.000 ± 2000 UE/mL) was obtained by using a manual mixer and the following conditions: Citrate-phosphate buffer 0.1 M, pH 7.4, 0.25 % Triton X-100, 2 % PVPP.
Purification of Apple PPO from Red Delicious apple
Purification of apple PPO was carried out by combination of different methods: dialysis (using a membrane with a MWCO 12000-14000), followed by ionic exchange chromatography on DEAE Sephadex A-50 and finally a hydrophobic chromatography (HIC) on Phenyl Sepharose CL-4B. The optimized protocol resulted in a purification factor of 37 with an activity recovery of 70 %. The purification process was evaluated by determining the specific activity at each step with CA and by SDS-PAGE electrophoresis (data not shown). The specific activity and the electrophoretic pattern were evaluated in every eluted fraction of both chromatographic steps involved in the optimized protocol. Therefore the molecular weight of the monomer of the enzyme was calculated to be 65 kDa, which is in concordance with bibliographic references [22-24]. An IEF was performed determining that the PI of the enzyme was 5.9.
Characterization of purified PPO from Red Delicious apple
Purified PPO was characterized to facilitate the study of enzyme´s inhibition under optimal and stable conditions. The optimum conditions for PPO activity were temperature between 20-37 °C and pH between 4.5-4.7. Apple enzyme showed good stability with temperature, since 100 %, 55 % and 38 % of the initial activity was recovered after 24 h at 23 °C, 35 °C and 50 °C respectively. The best range of pH stability after 24 h at room temperature was 4.4-4.7. Shelf stability was performed for 2 weeks at 4 °C and pH 4.7. The stability increased with the degree of purification, since 67 %, 90 % and 100 % of residual activity were recovered in the extract, partial and finally purified enzyme, respectively. Under the assessed conditions, apple PPO showed similar affinity towards CA and 4-MC, since KM values were 5.6 mM and 6.3 mM respectively.
b-cyclodextrin effect on PPO
The effect of β-CD on apple PPO was assayed by measuring enzyme activity in presence of increasing amounts of this oligosaccharide using CA and 4-MC as substrates (Figure 4).
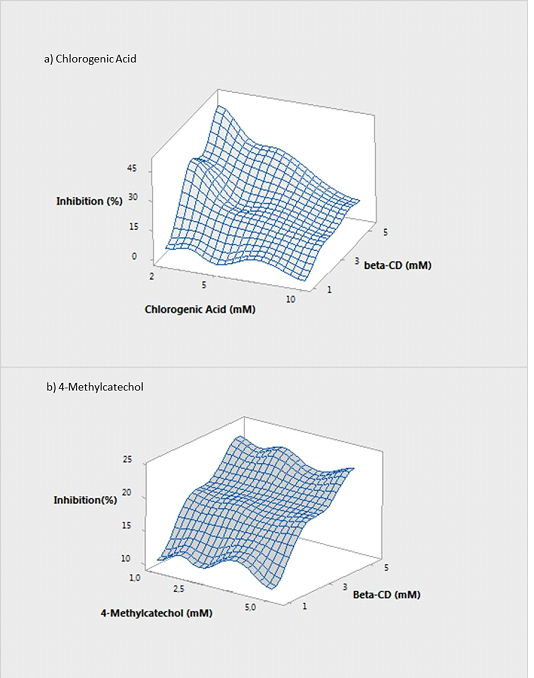
Figure 4. Effect of β-CD on PPO activity
Surface plot with different substrates: a) Chlorogenic acid (CA) and b) 4-Methyl Catechol (4-MC) at different concentrations were carried out.
Percentage of inhibition was calculated as follow:
I (%)= 100 – (AR/ AC x 100)
AR = Residual PPO activity after antibrowning agent addition
Ac= Control PPO activity (sample without antibrowning agent)
In order to compare the effect of β-CD and polyphenol concentration on the inhibition process and to establish if there was an interaction between them, a Two-way ANOVA of the general linear model was performed. From the ANOVA, β-CD concentration, CA concentration and the interaction of both factors had a significant effect (p < 0.05) on PPO inhibition. The Fisher F test showed high significance indicating that the fit of the model was very good (R-square 99.56 %). Figure 4a showed that, under the assessed conditions, the inhibition degree increased both by decreasing CA concentration or increasing the amount of β-CD. It was observed that the highest β-CD concentration (5 mM) and the lowest amount of CA (2 mM) allowed a reduction of apple PPO activity by more than 50 % of its initial value. High CA concentrations (eg.10 mM) could not be completely encapsulated and the residual CA concentration could be enough to maintain a high enzymatic activity (KM,CA = 5.6 mM) decreasing the inhibition degree.
When 4-MC was used as a model substrate, the Two-way ANOVA showed a different behaviour since only β-CD concentration had a significant effect on PPO inhibition (p < 0.05). This result was in concordance with Figure 4b, where the degree of inhibition at a selected β-CD concentration was not affected by substrate concentration.The lower inhibitory effect observed for 4-MC (compared with CA) could be explained by the different affinity of β-CD for this polyphenol. This outcome is in agreement with the reported dissociation constants (KD) of the complexes β-CD-Phenol for CA and 4-MC, 2.5 and 18.0 mM respectively [6].
The inhibitory effect of β-CD on apple PPO was more evident on purified enzyme than on raw extract because natural polyphenols were removed during the purification process, so they would not compete with exogenous substrates (CA and 4-MC) for the added β-CD (Data not shown).
Thiol-cyclodextrin effect on PPO
It was previously reported that Thiol-CD could behave as dithiotreitol (an aliphatic reducing agent) for controlling enzymatic browning [10]. Since copper (II) ions from the active site of PPO have an essential role for keeping its catalytic activity, they could be reduced by the derivatized CD giving an inactivated enzyme. Moreover, SH groups from Thiol-CD could also react with the o-quinones generated by the oxidation of polyphenols, giving colorless adducts, diminishing the production of brown pigments.
In order to demonstrate that an additional mechanism could be involved, Thiol-CD effect on apple PPO was studied, using CA and 4-MC as substrates. Both polyphenols were assessed at 10 mM concentration, almost twice of the KM value for each substrate
In the same way as β-CD, Thiol-CD had more affinity towards CA than for 4-MC. Furthermore, PPO inhibition increased with the derivatized CD concentration, while for 4-MC there was no effect until the maximum Thiol-CD concentration (45 µM) was assessed (Figure 5). These results proved that another mechanism was running in parallel with the reduction process, since the study was carried out under the same conditions for both polyphenols. Thus, it could be possible to assume that Thiol-CD behave as the unmodified β-CD and could also control PPO activity by the encapsulation of its substrates.
Moreover, when CA (10mM) was used as substrate, 100 % of inhibition could be achieved using 45 µM Thiol-CD (Figure 5) while under the same conditions, 5 mM β-CD gave only 11 % (Figure 4). Therefore, almost 100 times less concentration of Thiol-CD gave an increase of nearly 90% in antibrowning capacity, confirming that this derivatized CD had more than one mechanism for controlling enzymatic browning.
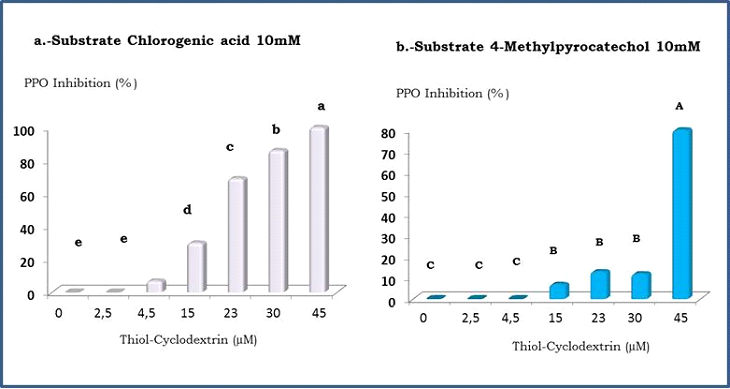
Figure 5. Effect of Thiol-CD on PPO activity
Studies with two substrates : a) Chlorogenic acid (CA) 10 mM b) 4-Methyl Catechol (4-MC) 10mM.
Results represent averages of at least three experiments. The same letter indicates no significant difference at the p≤ 0.05 level according to Tukey’s test
Table 1 shows the effect of β-CD and Thiol-CD on fresh sliced apple. The antibrowning capacity of these agents and the resulting increase on the shelf stability of apple slices were evaluated by measuring L*, a* and b*, CIELab parameters, after 2 h and 24 h [25].
Table 1.- Colorimetric determination of treated apple pulp with antibrowning agents
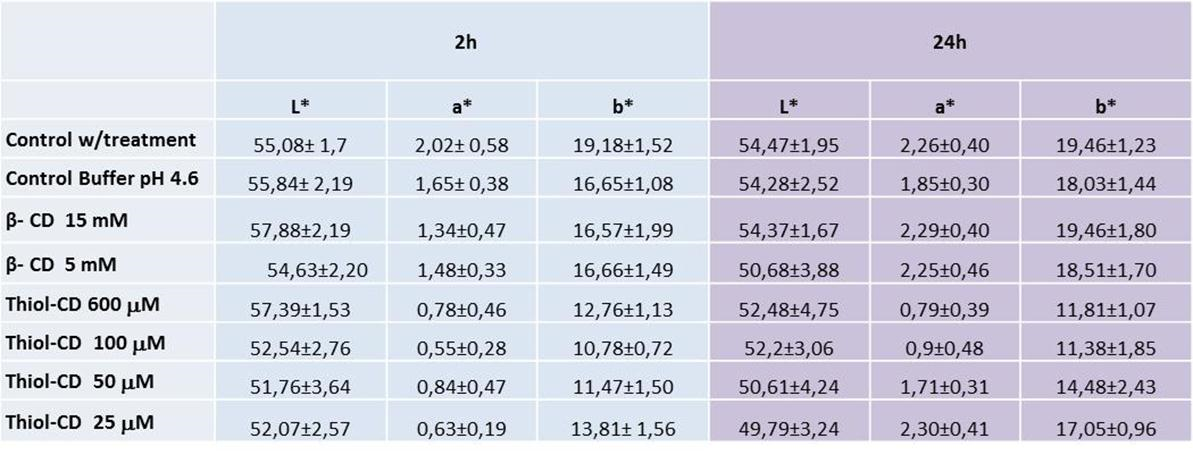
Three measurements were taken at three different areas of sliced apple (slice extremes and middle).
A decrease in a* was found for Thiol-CD, corresponding to a reduction in red color and therefore a decrease in enzymatic browning. Moreover a reduction in yellow color (decrease in b*) confirmed the same effect, meanwhile L* did not show an important disparity.
Two hours after slicing, even the smallest concentration of Thiol-CD assayed (50 μM), was effective for reducing browning in sliced apple. For keeping the appearance of fresh apple for longer periods it was necessary to increase Thiol-CD concentration. Over 100 μM Thiol-CD the values of a* and b* parameters after 24 h were almost unchanged, indicating that oxidative processes were completely stopped. These results showed that full PPO control on fresh apple required more Thiol-CD than in the case of isolated PPO. Diffusional problems on sliced apple could explain this behavior. On the other hand, 15 mM β-CD was effective after 2 h, however, after 24 h, CIELab parameters increased up to the same level as the ones for the controls (Table 1). Besides, the antibrowning effect of β-CD and Thiol-CD (under maximum concentration and 24 h incubation) can be directly visualized onto Red Delicious apple slices (Figure 6).
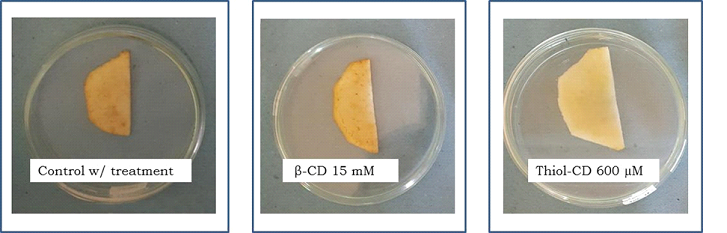
Figure 6. Effect of antibrowning agents on apple pulp
Red delicious apple: a) without treatment with antibrowning agent, b) β-CD 15mM and c)Thiol-CD 600mM. All samples were in Buffer acetate 0.1M pH5.0.
The antibrowning effect of β-CD and Thiol-CD was strongly dependent on the structure and concentration of the assessed substrate. Both inhibitors of PPO activity exhibited a better performance for chlorogenic acid (CA) than for 4-methypyrocatechol (4-MC) under the same conditions. When β-CD was used as an anti-browning agent, even under the best conditions (highest β-CD concentration and smallest CA concentration) only an inhibition degree of around 50% could be achieved. Both, modified and unmodified CDs could encapsulate polyphenols, but Thiol-CD demonstrated to be much more efficient than β-CD for controlling PPO activity, due to the presence of reducing thiol groups. The concentration needed for the same effect was much smaller than that needed for β-CD. This outcome would be of importance for food industry since lower levels of additive could be used to preserve minimally processed fruit and vegetables. Colorimetric determinations on apple slices confirmed that oxidizing processes were controlled by Thiol-CD treatment. This was in agreement with the behavior observed over the isolated PPO. In summary, Thiol-CD proved to be an excellent antibrowning agent with promising applications in the food industry.
Authors contributions
The postgraduate student Gabriela Peralta contributed to design and carry out the experimental work, she also participated in the data processing and its interpretation.
Dr. Carmen Manta and Dr. Karen Ovsejevi contributed to plan, design and supervise the experimental work, performing the analysis of the results and finally drafting the present manuscript.
Acknowledgements
We thank Dr Valerie Dee for linguistic revision.
Funding
We are grateful for financial support from Agencia Nacional de Investigación e Innovación (ANII), Project FCE 1-2014-1-103796 and from Programa para el Desarrollo de las Ciencias Básicas (PEDECIBA), Montevideo, Uruguay.
Altunkaya A, Gökmen V (2008). Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Latuca sativa). Food Chem. 107:1173-1179. doi:10.1016/j.foodchem.2007.09.046
View ArticlePilizota V, Subaric D (1998). Control of enzymatic browning of foods. Food Technol. 36 (3): 219-227
Podsedek A (2007). Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT - Food Sci. Technol. 40 (1): 1-11. doi: 10.1016/j.lwt.2005.07.023
View ArticleChisari M, Barbagallo RN,Spagna G (2008). Characterization and role of polyphenol oxidase and peroxidase in browning of fresh-cut melon. J Agric Food Chem. 56:132-138. doi:10.1021/jf0721491
View ArticleCheema S, Sommerhalter M (2015). Characterization of polyphenol oxidase activity in Ataulfo mango. Food Chem.171: 382–387. doi: 10.1016/j.foodchem.2014.09.011
View ArticleFayad N, Marchal L, Billaud C, Nicolas J (1997). Comparison of β-cyclodextrin effect on Polyphenol Oxidation catalyzed by purified Polyphenol Oxidase from different sources. J Agric Food Chem. 45: 2442-2446. doi: 10.1021/jf9607932
View ArticleLópez-Nicolás JM, Pérez-López AJ, Carbonell-Barrachina A and García-Carmona F (2007). Use of natural and modified cyclodextrins as inhibiting agents of peach juice enzymatic browning. J Agric Food Chem. 55: 5312-5319. doi: 10.1021/jf070499h
View ArticleFang Z X, Bhandari B (2010). Encapsulation of polyphenols A review. Trends Food Sci Technol. 21(10): 510-523
View ArticleAstray G, Gonzalez-Barreiro C, Mejuto JC, Rial-Otero R, Simal-Gándara J (2009). A review on the use of cyclodextrins in foods. Food Hydrocoll. 23: 1631-1640
View ArticleManta C, Peralta-Altier G, Gioia L, Méndez MF, Seoane G, Ovsejevi K (2013). Synthesis of a Thiol-β-cyclodextrin. A potential agent for controlling enzymatic browning in fruits and vegetables. J Agric Food Chem. 61: 11603−11609. doi:10.1021/jf403063s
View ArticleBravoa K, Osorio E (2016). Characterization of polyphenol oxidase from Cape gooseberry (Physalis peruviana L.) fruit. Food Chem.197:185-190. doi: 10.1016/j.foodchem.2015.10.126
View ArticleBatista-Viera F, Manta C, Carlsson J (1996). Covalent binding of thiols to thiolsulfinate-containing supports. Biotechnol. Appl. Biochem .24: 231-239
Broothaerts W, McPherson J, Li B, Randall E, Lane WD, Wiersma PA (2000).Fast Apple (Malus × domestica) and Tobacco (Nicotiana tobacum) Leaf Polyphenol Oxidase Activity Assay for Screening Transgenic Plants. J Agric Food Chem. 48: 5924–5928. doi: 10.1021/jf000599m
View ArticleSmith P K, Khron R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985). Measurement of protein using Bicinchoninic acid. Anal Biochem. 150: 76-85. doi: 10.1016/0003-2697(85)90442-7 90442-7
View ArticleSpagna G, Barbagallo RN, Chisari M, Branca F (2005). Characterization of a Tomato Polyphenol Oxidase and Its Role in Browning and Lycopene Content. J Agric Food Chem. 53 (6): 2032-2. doi: 10.1021/jf040336i
View ArticleChisari M, Barbagallo RN, Spagna G (2007). Characterization of Polyphenol Oxidase and Peroxidase and Influence on Browning of Cold Stored Strawberry Fruit. J Agric Food Chem. 55 (9): 3469-3476. doi: 10.1021/jf063402k
View ArticleFu Y, Zhang K, Wang N, Du J (2007). Effects of aqueous chlorine dioxide treatment on polyphenol oxidases from Golden Delicious apple. LWT- Food Sci. Technol . 40, 1362–1368. doi:10.1016/j.lwt.2006.11.001
View ArticleAlvarez-Parrilla E, De la Rosa LA, Rodrigo-García J, Escobedo-González R, Mercado-Mercado G, Moyers-Montoya E, Vázquez-Flores A, González-Aguilar GA (2007) Dual effect of β-cyclodextrin (β-CD) on the inhibition of apple polyphenol oxidase by 4-hexylresorcinol (HR) and methyl jasmonate (MJ). Food Chem.101 1346–1356. doi:10.1016/j.foodchem.2006.03.040
View ArticleBatista KA, Batista GLA, Alves GL, Fernandes KF (2014). Extraction,partial purification and characterization of polyphenol oxidase from Solanum lycocarpum fruits. J Mol Catal B Enzym. 102: 211-217. doi: 10.1016/j.molcatb.2014.02.017
View ArticleRocha AMCN, Morais AMMB (2001). Characterization of polyphenoloxidase (PPO) extracted from "Jonagored" apple. Food control 12: 85-90. doi: 10.1016/s0956-7135(00)00026-8 00026-8
View ArticleLe Bourvellec C, Le Quere JM, Snoner P, Drilleau JF and Guyot S (2004). Inhibition of apple Polyphenol Oxidase activity by Procyanidins and Polyphenol Oxidation products. J Agric Food Chem. 52, 122-130. doi:10.1021/jf034461q
View ArticleMurata M, Tsurutani M, Tomita M, Homma S, Kaneko K (1995).Relationship between apple ripening and browning: Changes in polyphenol content and polyphenol oxidase. J Agric Food Chem., 43, 1115-1121
View ArticleHaruta M, Murata M, Hiraide A, Kadokura H, Yamasaki M, Sakuta M (1998). Cloning genomic DNA encoding apple polyphenol oxidase and comparison of the gene product in Escherichia coli and in apple. Biosci Biotechnol Biochem. 62, 358-362
View ArticleJang JH, Moon KD (2011). Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 124:444-449. doi:10.1016/jfoodchem2010.06.052.
Wrolstad RE, Smith DE (2010). Color Analysis. In Nielsen S (ed). Food analysis, Fourth edition, USA, Springer, 578-585.
View Article