1. Gon-Sup Kim,
Tel: +82-55-772-2346, Fax: +82 55 772 2349; gonskim@gnu.ac.kr;
2. Sung Chul Shin,
Tel: +82-55-772-2346, Fax: +82 55 772 2349; 2130594@hanmail.net;
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 3 ISSUE: 4
Page No: 389-395
1. Gon-Sup Kim,
Tel: +82-55-772-2346, Fax: +82 55 772 2349; gonskim@gnu.ac.kr;
2. Sung Chul Shin,
Tel: +82-55-772-2346, Fax: +82 55 772 2349; 2130594@hanmail.net;
Jin Young Choi1,+ ∙ Soo Jung Lee2,+ ∙ Seong Min Kim3 ∙ Preethi Vetrivel3 ∙ Venu Venkatarame Gowda Saralamma3 ∙ Sang Eun Ha3 ∙ Kebede Taye Desta4 ∙ Won Sup Lee5 ∙ Hun Hwan Kim2 ∙ Gon-Sup Kim3,* ∙ Sung Chul Shin6,*
1 Department of Chemistry, Gyeongsang National University, Jinju, 52828, Republic of Korea
2 Department of Food and Nutrition, Institute of Agriculture and Life Science, Gyeongsang National University, Jinju 52828, Korea
3 Research Institute of Life Science and College of Veterinary Medicine, Gyeongsang National University, Jinju, 52828, Republic of Korea
4 Department of Chemistry, College of Natural and Computational Sciences, Mekelle University, P.O.Box: 231, Mekelle, Ethiopia
5 Department of Internal Medicine, Institute of Health Sciences and Gyeongnam Regional Cancer Center, Gyeongsang National University, Jinju, 52828, Republic of Korea
6 Research Institute of Life Science Gyeongsang National University, Jinju, 52828, Republic of Korea
+ These two authors contributed equally to this study.
Ireneusz Kapusta(ikapusta@ur.edu.pl)
Volker B%C3%B6hm(Volker.Boehm@uni-jena.de)
Kiran Thakur(kumarikiran@hfut.edu.cn)
Amin Shavandi(amin.shavandi@otago.ac.nz)
Gon-Sup Kim, Sung Chul Shin, Effect of Harvest Season on Polyphenol Profile and Antioxidant Activities of Seomae Mugwort (Korean Artemisia argyi H. L
The effect of the harvest season of polyphenol mixtures isolated from Seomae mugwort (SM, Korean Artemisia argyi H. Lé v. & Vaniot) plant was evaluated in terms of the content and antioxidant capacity. The results indicated a clear difference of total phenolic and individual polyphenolic contents in SM depending on harvesting time. The total polyphenol content was highest in SM collected in June (138.64 mg/kg) followed by that in May (125.34 mg/kg) and that in August (82.34 mg/kg). The antioxidant activity of the SM polyphenol mixture was measured in terms of assays like DPPH•, ABTS•+, superoxide anion radical-scavenging activities, and FRAP reducing power. The antioxidant activity order is roughly in accordance with the total polyphenol contents based on the harvest seasons. It was observed that the antioxidant activity depends mainly on the content of hydroxyl cinnamates and flavonoids, which are the major polyphenol compounds.
Keywords: Seomae mugwort, Korean Artemisia argyi, ∙ Antioxidant activity, ∙ Polyphenol, ∙ High performance liquid chromatography, Tandem mass spectroscopy,
The imbalance between free radicals and the body’s ability to detoxify or repair cells results in cell death and oxidative stress, causing extensive damage to biological molecules. In order to prevent such oxidative stress and damage, the search for new antioxidants from natural sources is always recommended in health (Kwak & Lee 2014). The mugwort plant, Artemisia argyi H. of the genus Artemisia, is a perennial herbaceous plant widely distributed in most of Eastern Asia. It has been used to treat abdominal pain, hematemesis, chronic hepatitis, anorexia, chronic gastroenteritis caused by sedation, convulsions, paralysis, and systemic stiffness as folk remedies in the Korean peninsula (Bae 2003; Lee et al. 2000). Seomae mugwort (Korean Artemisia argyi H. Lé v. & Vaniot, SM) is grown in Namhae-gun province, Republic of Korea (ROK), which has favorable conditions for cultivation, such as sea breezes and abundant sunshine (Ha et al. 2012). The genetic and morphological characteristics of the plant are different from Artemisia argyi, which is ubiquitous in East Asia and has been registered as a local-specific resource in the Korean Forest Service variety protection registration (No. 42, 2013, 09. 27) (Ha et al. 2015).
It has been well known that the A. argyi plant contains various types of phytochemicals, including polyphenols (Park et al. 2009; Carvalho et al. 2011). The polyphenols of the plant have been well characterized (Han et al. 2017). The polyphenols in the SM plant were characterized in our recent study, and about 14 polyphenol compounds have been reported: five hydroxycinnamates (two caffeoylquinic acid isomers, dicaffeoylquinic acid, 3,4,5‐O‐tricaffeoylquinic acid, calcelarioside A), eight flavonoids (6,8-di-C-glucosylapigenin, 6-C-arabinosyl-8-C-glucosylapigenin, two amentoflavone isomer, kaempferol-3-O-rutinoside, kaempferol-3-O-glucuronide, quercetin-dimethyl-ether, skullcapflavon Ⅱ), and one lignan (secoisolariciresinol) (Kim 2016). Polyphenols play an effective role as antioxidants that can scavenge reactive oxygen species (ROSs), thereby retarding the pathogenesis of age-related degenerative diseases, such as diabetes, cardiovascular disease, and cancer (Kim et al. 2016; Kim 2014; Song et al. 2016). Plant polyphenols not only determine the functional quality in terms of plant products but also serve as an important factor in their potential application in the fields of nutrition and pharmacology (Carvalho et al. 2011) and in the food industry to increase self-life (Wei et al. 2017), on which scientists have been concentrating with great interest. The chemical components and physiological activities of plants have been reported to differ depending upon the genotype, growth stage, and various aspects of environmental conditions, such as soil, climate, and light (Kim et al. 2014; Deepa et al. 2007; Cho and Chiang 2001). Although the maturity stage can affect the components and activities, little attention has been given to how the maturity stage does so (Pandino et al. 2012). In this regard, it may be worthwhile to investigate the effect of harvest season on active components; the resulting data will be useful for processing them as high-quality, functional products.
Herein, we determined to investigate the differences in the polyphenols of SM plants and their antioxidant activity over a period of four months (May to August) by using liquid chromatography-tandem mass spectrometry (LC-MS/MS).
Chemicals and reagents
All the reagents and standards used were analytical grade. Amentoflavone, apigenin, catechin, caffeic acid, flavone, kaempferol, and quercetin were purchased from Sigma‐Aldrich Co. (St Louis, MO, USA), recrystallized in methanol, and used as external standards. The purity of all standards was ascertained by LC to be > 99%. Reagents for the antioxidant activity assays were also purchased from Sigma‐Aldrich Co. All solvents used were obtained from Duksan Pure Chemical Co. Ltd (Ansan, Republic of Korea).
Plant collection, sample extraction, and preparation
The SM plants were collected between May and August of 2017 from Namhae-gun, Gyeongsangnam-do, Republic of Korea, dried under shade, and powdered. From each sample, 100 g were refluxed in 70 % methanol (1.5 L) for 20 h. The mixture was filtered through a Büchner funnel and concentrated to approximately 300 mL at reduced pressure at 35°C, using a rotary evaporator. The concentrated filtrate was washed with n-hexane (300 mL × 3) to remove nonpolar impurities. The filtrate was extracted using ethyl acetate (100 mL × 3), and dried over anhydrous MgSO4. The solvent was removed under reduced pressure. The sticky residue was placed on top of a silica gel solvent (40 × 2.5 cm) and eluted with ethyl acetate to eliminate highly polar impurities. The solvent was then removed to yield solids of polyphenol mixture (19.8% for May, 19.4% for June, 10.3% for July, and 17.6% for August); 1 mg/mL of each sample was prepared in 80% aqueous methanol, filtered, and taken for analysis.
HPLC and ESI-MS/MS Analysis
HPLC analysis was conducted using a 1200 series HPLC system (Agilent Technologies, Palo Alto, CA, USA) with a Multiple Wavelength Detector (MWD) set at 254, 280, 320, or 360 nm. The column used was Prontosil C18 column (4.6 × 250 mm, 5 μm, Bischoff Co., Leonberg, Germany) which was set at 35°C. A binary mobile phase system consisting of 0.5% formic acid (solvent A) and methanol (solvent B) was used. The gradient condition used was 0-10 min, 15% B; 10-15 min, 15-20% B; 15-25 min, 20% B; 25-30 min, 20-25% B; 30-60 min, 25-45% B; 60-65 min, 45-70% B; 65-70 min, 70-15% B. The flow rate was maintained at 1 mL/min, and the volume of sample injection used was 10 μL in each experiment. The ESI-MS/MS analysis was conducted using a 3200 QTrap tandem mass system (Sciex LLC, Framingham, USA), and the obtained data was analyzed using BioAnalyst™, version 1.4.2.
Quantification method
Polyphenol mixtures from each sample (as per collection month) were quantified using LC-UV chromatograms (at 280 nm) with seven selected standards (amentoflavone, apigenin, catechin, caffeic acid, flavone, kaempferol, and quercetin) according to the method reported elsewhere (Kim 2016).
Antioxidant activities
The antioxidant activity of the polyphenol mixtures from each sample was evaluated in terms of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, [2,2`-azinobis-(3-ethylbenzo-thiazoline-6-sulfonate)] (ABTS) cation-radical scavenging activity, superoxide anion radical (O2-) scavenging activity, and ferric-reducing antioxidant potential (FRAP) assay according to the previously reported methods (Choi et al. 2018; Liu et al. 1997).
Statistical analysis
Data were analyzed statistically by one-way analysis of variance (ANOVA), and the appropriate mean values were separated using the Duncan’s multiple range test at p < 0.05.
LC-MS/MS analysis
LC-MS/MS analysis was conducted under the conditions described in a previous study (Kim 2016; the LC-chromatograms obtained for each sample for harvesting time are shown in Figure 1. The 14 peaks for the polyphenols are labeled in order based on their retention time (tR), and structural characterization of each polyphenol was done using [M-H]- data, MS/MS fragmentations peaks, and available literature data. The 14 peaks were characterized as five hydroxycinnamtes (caffeoylquinic acid isomer (1), caffeoylquinic acid isomer (6), dicaffeoylquinic acid (10), 3,4,5‐O‐tricaffeoylquinic acid (11), and calcelarioside A (14)), eight flavonoids (6,8-di-C-glucosylapigenin (2), 6-C-arabinosyl-8-C-glucosylapigenin (3), amentoflavone isomer (5), amentoflavone isomer (7), kaempferol-3-O-rutinoside (8), kaempferol-3-O-glucuronide (9), quercetin-dimethyl-ether (12), and skullcapflavon Ⅱ(13)), and one lignin (secoisolariciresinol (4)). Their structural characterization has been discussed in detailed in Kim (2016).
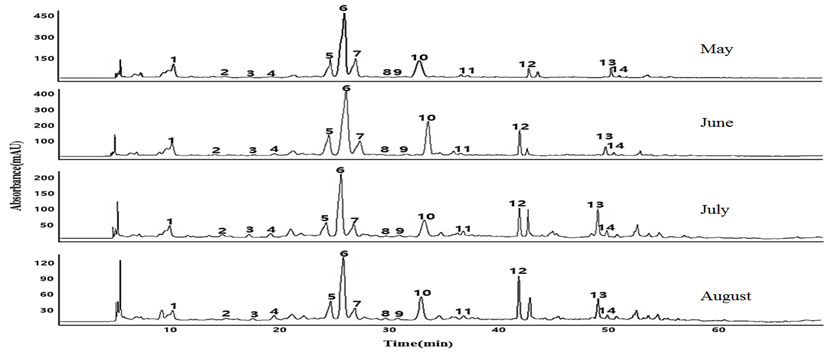
Figure. 1. LC-chromatograms of polyphenols from Seomae mugwort for each harvest period.
Quantification method
Quantification of the characterized polyphenols was done using the calibration curves obtained from representative external standards, as described in the earlier study (Kim 2016). Satisfactory validation data were obtained for all the considered parameters. All 14 polyphenols were detected in each sample with significant differences in concentration. The concentration of each polyphenol in SM with respect to the different harvest periods is summarized in Table 1. The highest total polyphenol content was found in samples collected in June, followed by that in May, and the least was found in August. There were also significant differences in the content of individual polyphenols during the sample harvesting time. For example, for compound 13, the highest content was observed in July, the least in May. For compound 8, the highest content was observed in August, the least in May. In each case, caffeoylquinic acid derivatives (1, 6, 10) and amentoflavone isomers (5, 7) are the compounds that were identified as abundant. Overall, the result indicated clear differences of total and individual polyphenol contents in SM in terms of harvesting time.
Table 1. Concentration of polyphenols in Seomae mugwort with respect to harvesting time
|
Compd. No |
Content with respect to harvest periods (mg/kg of shade dried sample) |
|||
|
May |
June |
July |
August |
|
|
1 |
7.25±0.41D |
6.61±0.30C |
5.59±0.17B |
3.30±0.11A |
|
6 |
58.91±0.54C |
63.27±1.43D |
38.06±0.45B |
30.54±0.21A |
|
10 |
19.90±0.31C |
23.38±0.61D |
14.14±0.29 B |
13.02±0.24A |
|
11 |
0.84±0.11A |
1.05±0.05 B |
2.21±0.08D |
1.20±0.03C |
|
14 |
0.65±0.04A |
0.76±0.07 B |
1.95±0.08D |
0.97±0.02C |
|
Total hydroxyl cinnamates |
87.55±0.28C |
95.07±0.49D |
61.95±0.21B |
49.03±0.12A |
|
2 |
0.92±0.03D |
0.47±0.02B |
0.67±0.04C |
0.31±0.04A |
|
3 |
0.30±0.02A |
0.58±0.02B |
1.54±0.04D |
0.72±0.02C |
|
5 |
12.18±0.51C |
15.56±0.83D |
9.44±0.40B |
7.70±0.07A |
|
7 |
15.83±1.17D |
12.85±0.72C |
9.28±0.54B |
6.21±0.06A |
|
8 |
0.21±0.01A |
0.30±0.01B |
0.39±0.01C |
0.78±0.09 D |
|
9 |
0.11±0.01NS |
1.13±0.03 |
2.93±4.34 |
0.78±0.03 |
|
12 |
3.67±0.11A |
7.82±0.29B |
8.01±0.05B |
9.27±0.04C |
|
13 |
3.59±0.29A |
3.68±0.13A |
10.16±0.21C |
5.61±0.06B |
|
Total flavonoids |
36.81±0.27B |
42.39±0.26C |
42.41±0.66C |
31.38±0.05A |
|
4 |
0.97±0.01A |
1.17±0.04B |
2.16±0.15D |
1.92±0.05C |
|
Total |
125.34±1.31C |
138.64±2.84D |
106.53±4.84 B |
82.34±0.33A |
A-D Means with different superscripts in the same column are significantly different at P<0.05 by Duncan's multiple range tests.
NS: not significant
Antioxidant activities
The antioxidant activity of the plant samples differed with various experimental conditions, such as solvent, temperature, pH, and radical type; therefore, for reliable evaluation, an assay combining a variety of in vitro methods is required (Choi et al. 2018). In this study, the antioxidant activity of the polyphenol mixture isolated from the Seomae mugwort was assayed by means of DPPH•, ABTS•+ scavenging, superoxide anion radical-scavenging activities, and reducing power by FRAP assay. The results obtained from the assays are summarized in Table 2. Effective concentration (EC) values were calculated from regression lines using five different concentrations (50 to 1000 μg/mL), and radical scavenging activity was calculated as the sample concentration required to scavenge 50% of the radical species. FRAP activity was measured at 200 μM equivalent weight of FeSO4·7H2O. Because EC and reducing power are relatively inverse to the antioxidant activity of the compound, the lower values indicate higher antioxidant activity (Choi et al. 2018).
The antioxidant activity measured via the DPPH• method provides evidence similar to that found from by the ABTS•+ and superoxide anion radical scavenging activities. The antioxidant activity of the polyphenol mixture increased in a dose-dependent manner (data not shown). The antioxidant activity was significantly higher in samples harvested on June, followed by May, July, and August, except for the superoxide anion radical scavenging (Table 2), for which the sample harvested on June to July (269.05~293.91 μg/mL) was the highest, followed by May (357.25 μg/mL) and August (518.70 μg/mL). This activity order is roughly in accordance with the total polyphenols contents according to the harvest seasons. It depends on the contents of the hydroxyl cinnamates and flavonoids, which were the major polyphenol components (see Table 1). Among the hydroxyl cinnamates and flavonoids, caffeoylquinic acid derivatives and amentoflavones are especially known to possess various pharmacological activities, including antioxidant, anti-viral, anti-depressant, and anti-inflammatory effects (Miyamae et al. 2011; Zhang et al. 2011). Considering the fact that caffeoylquinic acid derivatives and amentoflavones are major components and their well-known pharmacological activities, the antioxidant activity in SM should be attributed to them.
Table 2. Antioxidant activities of polyphenol mixtures from Seomae mugwort with respect to harvest periods
|
|
Antioxidant activities |
|||
|
|
DPPH radical scavenging* (μg/mL) |
ABTS radical scavenging* (μg/mL) |
Superoxide anion radical scavenging* (μg/mL) |
Reducing power by FRAP** (μM) |
|
May |
147.74±5.03A |
177.48±4.03 B |
357.25±46.42 B |
113.49±1.88 B |
|
June |
137.87±7.94 A |
166.19±1.77 A |
269.05±24.36 A |
87.96±2.02 A |
|
July |
282.15±5.96 B |
286.19±4.67 C |
293.91±38.43 A |
128.32±3.29 C |
|
August |
423.51±25.34 C |
387.83±8.27 D |
518.70±51.05 C |
211.74±6.62 D |
A-DMeans with different superscripts in the same column are significantly different at p < 0.05 by Duncan's multiple range tests.
DPPH•, α,α-diphenyl-β-picrylhydrazyl; ABTS•+, 2-2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
Each value represents the mean ± standard deviation (n = 4).
*Effective concentration (EC, μg/mL) values were calculated from regression lines using five different concentrations (50 to 1000 μg/mL), and was defined as 50% scavenging activity for DPPH, ABTS, and superoxide anion radical.
**EC (μM) value for the reducing power by FRAP assay was measured at 200 μM equivalent weight of FeSO4·7H2O.
The differences in content and antioxidant activities of polyphenols in the SM plants collected over a period of four months (May to August) were analyzed. A total of 14 polyphenols were detected. The samples collected in June (138.64 mg/kg) were found to be the richest in polyphenols and showed the highest antioxidant activities. Hence, high-quality, functional products can best be made from SM plants harvested in June.
This research was supported by the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (no. 2012M3A9B8019303 and 2017R1A2B4003974).
Carvalho IS, Cavaco T, Brodelius M (2011) Phenolic composition and antioxidant capacity of six artemisia species. Ind Crops Prod 33:382-388
View ArticleCho YH, Chiang MH (2001) Essential oil composition and antibacterial activity of Artemisia capillaris, Artemisia argyi, and Artemisia princeps. Korean J Int Agric 13:313-320
Choi JY, Desta KT, Lee SJ, Kim YH, Shin SC, Kim GS, Lee SJ, Shim JH, Abd El-Aty AM (2018) LC-MS/MS profiling of polyphenol-enriched leaf, stem, and root extracts of Korean Humulus japonicus Siebold & Zucc and determination of their antioxidant effects. Biomed Chromatogr. doi: 10.1002/bmc.4171
View ArticleDeepa N, Kaur C, George B, Singh B, Kapoor HC (2007) Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. J Food Sci Technol 40:121-129
View ArticleHa GJ, Lee DS, Seung TW, Park CH, Park SK, Jin DE, Kim NK, Shin HY, Heo HJ (2015) Anti-amnesic and neuroprotective effects of Artemisia argyi H. (seomae mugwort) extracts. Kor J Food Sci Technol 47:380-387
View ArticleHa GJ, Lee YH, Kim NK, Shon GM, Rho CW, Jeong HR, Heo HJ, Jeong CH (2012) Nutritional chemical composition in the different parts of Artemisia argyi H. J Agric Life Sci 46:155-164
Han B, Xin Z, Ma S, Liu W, Zhang B, Ran L, Yi L, Ren D (2017) Comprehensive characterization and identification of antioxidants in Folium Artemisiae argyi using high-resolution tandem mass spectrometry. J Chromatogr B 1063:84-92 PMid:28850890
View Article PubMed/NCBIKim EJ, Kim GT, Kim BM, Lim EG, Ha SH, Kim SY, Kim YM (2016) Apoptotic effect of extract from Artemisia annua Linné by kt/mTOR/GSK-3β signal pathway in Hep3B human hepatoma cells. J Life Sci 26:764-771
View ArticleKim JY, Cho JY, Lee KD, Kim SJ, Choi KC, Ham KS, Park KH, Moon JH (2014) Change of phenylpropanoic acid and flavonol contents in different growth stage of glasswort (Salicornia herbacea L.). Food Sci Biotechnol 23:685-691
View ArticleKim MR (2016) Physicochemical properties and liver-protecting effect of Seomaeyakssuk (Artemisia argyi H.). Ph.D. Thesis, Submitted to Gyeongsang National University, Korea
View ArticleKim SH (2014) Optimization of ethanol extraction conditions for Artemisis capillaris effective components using response surface methodology. J Korean Soc Food Sci Nutr 43:741-748
View ArticleKwak CS, Lee JH (2014) In vitro antioxidant and anti-inflammatory effects of ethanol extracts from sprout of evening primrose (Oenothera laciniata) and gooseberry (Actinidia arguta). J Korean Soc Food Sci Nutr 43:207-215
View ArticleLee SD, Park HS, Kim DW, Bang BH (2000) Bioactive constituents and utilities of Artemisia sp. as medical herb and food stuff. Korean J Food Nutr 13:490-505
Liu F, Ooi VEC, Chang ST (1997) Free radical scavenging activity of mushroom polysaccharide extracts. Life Sci 60:763-771 00004-0
View ArticleMiyamae Y, Kurisu M, Han H, Isoda H, Shigemori H (2011) Structure-activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem Phar Bull (Tokyo) 59:502-507
View ArticlePandino G, Lombardo S, Williamson G, Mauromicale G (2012) Polyphenol profile and content in wild and cultivated Cynara cardunculus L. Ital J Agron 7:254-261
View ArticlePark MH, Kim MJ, Cho WI, Chang PS, Lee JH (2009) Effects of treatments on the distribution of volatiles in Artemisia princeps Pampan. Korean J Food Sci Technol 41:587-591
Song Y, Desta KT, Kim GS, Lee SJ, Lee WS, Kim YH, Jin JS, Abd El-Aty AM, Shin HC, Shim JH, Shin SC (2016) Polyphenolic profile and antioxidant effects of various parts of Artemisia annua L. Biomed Chromatogr 30:588-595 PMid:26285146
View Article PubMed/NCBIZhang YX, Li QY, Yan LL, Shi Y (2011) Structural characterization and identification of biflavones in Selginella tamariscina by liquid chromatography-diode-array detection/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 25:2173-2186 PMid:21710597
View Article PubMed/NCBIZhang YY, Zhang F, Thakur K, Ci AT, Wang H, Zhang JG, Wei ZJ (2017) Effect of natural polyphenol on the oxidative stability of pecan oil. Food Chem Toxicol doi:10.1016/j.fct.2017.10.001
View Article