Jie Guan, Linhui Ma ,Ruolan Jiang, Hengping Shu, Jie Zhang
Department of Parasitology, School of Basic Medical Sciences, Xiangya School of Mdicine, Central South University, Changsha, China
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 7
Page No: 852-859
Jie Guan, Linhui Ma ,Ruolan Jiang, Hengping Shu, Jie Zhang
Department of Parasitology, School of Basic Medical Sciences, Xiangya School of Mdicine, Central South University, Changsha, China
Shifeng Wang(shifeng_15@163.com)
Charoonroj Chotwiwatthanakun(charoonroj.cho@mahidol.ac.th)
Baojun Tang(Tangbj@ecsf.ac.cn)
Jie Guan, Linhui Ma ,Ruolan Jiang, Hengping Shu, Jie Zhang, Identification and occurrence of Anisakis larvae from Marine Fish in Changsha City, Hunan province, China (2019)Journal of Food Science & Technology 4(7)p:852-859
Anisakis spp. (Nematoda: Anisakidae) parasitize a wide range of marine animals, mammals serving as the definitive host and different fish species as intermediate or paratenic hosts. The present study was performed to investigate the infection status of the third stage larvae (L3)on Anisakis in marine fishes for sale in Changsha City, Hunan province, China. Marine fishes were randomly collected from markets in Changsha City from January to July in recent years, and then classified. And a total of 142 fish including 8 species of fish were investigated ,including ribbonfish 15 ,small yellow croaker 30, saury 20, turbot 5, bummalo 20, sardines 30, Cuttlefish 10 and yellow teeth 12. we carefully dissected and examined each individual's body cavity, viscera organs, back, abdomen, fish tail and recorded the infection rates of nematodes. The staining nematodes by hydrochloric acid carmine red were carried conventional morphological identification under an optical microscope, seven nematodes were randomly selected to extract genomic DNA for molecular biological identification. The infection rate of the third stage larvae (L3) of Anisakis was observed, respectively 33.3% in ribbonfish(5/15), 10% in small yellow croaker (3/30),25% in saury(5/20), 75% in bummalo (15/20). The infection of the third-stage larvae of the genus Heterodera in the fish market in Changsha is relatively serious, and the degree of infection has a certain relationship with the type and body parts of the fish. Therefore, this will give some hints and guidance for future health quarantine and fishery production, processing and export.
Keywords: Changsha City; marine fish; third-stage larvae of anisakis; parasitic diseases
The intermediate host of the Anisakis is mostly marine fish, which is distributed all over the world. Case reports of this disease have been reported in more than 20 countries including Japan, the Netherlands, the United Kingdom, France, Germany and the Pacific [1, 2]. Among them, more than 14,000 human cases have been reported in Japan. The main reason is that residents of these countries like to eat salted sea fish, or like to eat raw sea fish fillets, fish liver, caviar or squid for food and drink, which made the disease become a natural foci of marine disease[3]. In China, although there have been no case reports so far, it was found that the infection rate of Anisakid was high in the domestic selling-fish market, especially in small fish’s muscle or organs such as bummalo, small yellow croaker, ribbonfish. 25 types of fish is infected in East Sea and the Yellow Sea and 15 types of fish is infected in northern Gulf [4]. The study found that under the conditions of -2 ° C and -8 ° C for 96 hours, the Anisakis still have the ability to invade the rat tissue, and the ability disappears after 14 hours at -20 ° C. In Japan, 12 cases of Anisakis larvae were infected by eating the genitals of raw salmon. Among them, mild cases showed gastrointestinal discomfort, and severe cases suddenly occurred, similar to surgical acute abdomen[5, 6]. The performance was sudden pain in the epigastrium a few hours after eating with nausea, vomiting, diarrhea. It was visible by fiber gastroscope in severe patients,such as mucosal edema, hemorrhage, erosions, ulcers,and even tumor samples appeared on advanced patients’ gastric wall[7]. In Changsha, the residents have a very heavy taste , like to eat a variety of fresh foods, in particular, like to eat visceral hotpot and directly soaked animal meat by wine , resulting in a greater chance of infection. The United Nations food and agriculture organization (FAO) pointed that Anisakis was one of the most important biological hazard factors in the seafood safety and quality risk assessment in 2004,so it is of important public health significance to the prevention of Anisakiasis that the infection status of Anisakis was investigated in common marine fishes for sale in Changsha City.
2.1. Sampling Methods and Fishes
A three-stage cluster random sampling method is carried out from January to July in recent years. The first stage is to randomly select a large market in a region (Furong District) from 9 districts and counties (cities) in Changsha. In the second stage, a large-scale market (Mawangdui Wholesale Market) was extracted from this district and by simple random sampling. In the third stage, 10 stores were randomly selected from the aquatic areas of the large market by simple random sampling, and a total of 142 fish were purchased including eight types, respectively ribbonfish 15 ,small yellow croaker 30, saury 20, turbot 5, bummalo 20, sardines 30, Cuttlefish 10 and yellow teeth 12. Most of these fish was frozen transported to the market after the capture by the fishermen next to yellow sea and east sea, a small part was aquaculture. It was pointed from the literature that Anisakis was distributed in the gastrointestinal submucosa, so we divided the fish body in order to examine the infection status of fish. This division is slightly different with the definition of various organs in anatomy. Head - the trailing edge of the snout to the rear edge of the gill cover, not including the pharynx and esophagus; Trunk - After the head, before the cloaca (removing the internal organs) ;Back (figure 1a) and abdominal (figure 1b) is bounded to the bones of the fish; Tail -- after the cloacal orifice area (figure 1 c); Esophageal and gastric wall outside, inside the body cavity mucosa as the body cavity; esophageal and gastric bowel for gastric ministry(See Figure 1).
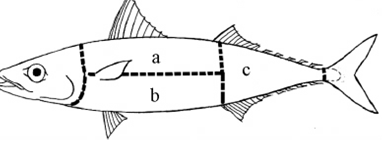
a: the back muscles; b: abdominal muscles; c: the tail muscle
Figure 1. Schematic diagram of division of fish parts of Pneumatophorus japonica
2.2 The main equipment and reagents
2.2.1 The main equipment
Optical microscope (Japan QLYMPUS), low-speed desktop centrifuge (Pingfan instrument TDZ5-WS type), high-speed desktop centrifuge (Shanghai Anting Scientific Instrument Flying Pigeon series AWke TGL-16B), PCR instrument (American Bio metra company Tpersonal type ), electrophoresis (Beijing LiuYi instrument DZ-600 type), UV gel imaging Miriam (US GelDoc-IT companies), pipettes (Germany EPPENDORF company), water systems (Millpore Purification column, Milli - Q), Clean Benches (Suzhou Antai Airtech companies), Vertical Pressure Steam Autoclave (Shanghai, China Nuclear Medical Instrument Co. line), electronic balance (the Haimeitele - Toledo Instruments Analytical Balance), Joyoung cooker, microwave power cards.
2.2.2 Reagent and medicine
Protease K (Shanghai biological engineering co., LTD), TIANamp Genomic DNA Kit DP304-02 pillar type blood/organization/cell Genomic DNA extraction Kit (Shanghai biological engineering co., LTD), DNA Marker DL2000bp DNA Ladder (Tiangen biochemical technology co., LTD), Hydrochloric carmine dye (provided by Yanganwei professor, Parasitology, xiangya school of medicine, Central South university),Specimens fixative (70%ethanol 45ml, 40%formaldehyde 3.5ml, acetic acid 1.5ml),acid alcohol (80%ethanol 100ml and concentrated hydrochloric acid 2ml).
2.2.3 Electrophoresis buffer
10 × TAE (pH8.3): Tris 108g, boric acid 55g, EDTA (0.5mol / L) 20Ml
2.3 Sample processing, morphological identification
We cut the body cavity from the cloaca, carefully checked it and gastrointestinal and so on, torn along the direction of the muscle fibers. If we found nematodes, we would pick parasites to the corresponding number of petri dish, wash with ultrapure water, place them to fixative in order to fix the parasite, observe one by one under the microscope, reference to the literature, We conducted a preliminary identification of the parasite according to the characteristic of forms, the head and tail in order to determine classification status.
2.4 DNA extraction and PCR amplification
The whole genome DNA of the parasites was extracted according to DNA extraction Kit instructions and was saved for the backups at -20℃. The seven parasites removed from the fixative were washed repeatedly with deionized water for 2-3 hours and then placed in a new 1.5 ml EP and labeled on an EP tube. DNA extraction according to TIAN amp Genomic DNA Kit DP304-02 Blood/tissue/cell genomic DNA extraction kit instructions. The four different pairs of primers were synthesized by Jinsirui Biotechnology Co., Ltd. In Nanjing separately, they are of Anisakis simplex,Pseudoterranova, Contraceacum and Hysterothylacium. The PCR reaction system were: 10 × PCR buffer (Mg2 +) 5μl , deoxyribonucleotide triphosphate (dNTP, 2.5 mmol / L) 1μl, upstream primer (10μmol / L, each of four kinds was 1μl) 4μl, downstream universal primers 8μl, Taq DNA polymerase (5U / μl) 1μl, DNA template 2.0μl, added ddH2O to 50μl. And the reaction conditions were: 95 ℃ for 5 min, 95 ℃ for30 s, 56 ℃ for 30 s, 72 ℃ for 1 min, a total of 34 cycles, 72 ℃ extension for 10 min, and finally stored at 4 ℃.Additionally, the expected amplified fragment size is approximately 1000 bp.
3.1 Sample detection and morphological identification
The detected nematodes were linear, colorless or milk white, and parts of them were wrapped light-yellow membrane in the outer. The live, moving such as earthworms, could be clearly seen white dots with the naked eye in the end, which is a small stomach. After being fixed and transparent, the total length of the worms is 1 to 3 cm. Under the microscope, their drilling teeth on the head barbed the ventral in the shape of partial triangle, no neck mastoid, no intestinal caecum and no gastric caecum, small stomach is elongated, gastrointestinal junction was butt or miter (Figure. 2A), a fantail in the end (Figure 2B). The above characteristics are consistent with the morphological characteristics of the third-stage larvae of the genus Anisakis, indicating that the nematodes collected from the fish are all the third-stage larvae of the genus Anisakis , and all of them are Type I larvae belonging to the genus Anisakis. However, this method cannot completely identify the species, and it can be further identified by molecular biological methods such as MAE , PCR-RFLP , PCR-SSCP , ribosomal DNA sequencing.

Figure 2. Photoelectron micrograph of anisakis. A:The front view of the worm head shows the drill head under the optical microscope. B:Rear view of fan tail under optical microscope
3.2 The infection status of Anisakis in marine fish
A total of 142 fish and 8 species of fish were investigated, including ribbonfish 15, small yellow croaker 30, saury 20, turbot 5, bummalo 20, sardines 30, cuttlefish 10 and yellow teeth 12 . The infection rate of the third stage larvae (L3) of Anisakis was respectively 33.3% in ribbonfish(5/15), 25% in saury(5/20), 10% in small yellow croaker (3/30), 75% in bummalo (15/20).The anisakids was not found in sardines, turbot ,Cuttlefish and yellow teeth. Among them 14 nematodes were found in ribbonfish, 10 in saury, 5 in small yellow croaker , 22 in bummalo,0 in turbot, Cuttlefis, yellow teeth and sardines(Table. 1). More categories and number were detected in saury. One of saury was detected a typical Anisakis and 2 black nematodes with the same size, others were checked out red nematodes with stripe shape from one to many, about 1 ~ 3 cm length, species is not yet clear.
Table 1. The detection rate of Anisakis larvae of several fish in Changsha market
|
|
quantity(tail) |
positive number(tail) |
the number of parasites detected |
|
ribbonfish |
15 |
5 |
14 |
|
saury |
20 |
5 |
10 |
|
small yellow croaker |
30 |
3 |
5 |
|
turbot |
5 |
0 |
0 |
|
cuttlefish |
10 |
0 |
0 |
|
yellow teeth |
12 |
0 |
0 |
|
bummalo |
20 |
15 |
22 |
|
sardines |
30 |
0 |
0 |
3.3 The infection status in different parts of common fish
The study found that the infection rates of different fish species and parts in the Changsha market are different. Larval infections are most common in body cavities and internal organs. The saury is mainly found in the underarm area, and the ribbonfish, bummalo are detected under the mesentery. As shown in table. 2.
Table 2. Detection of Anisakis larvae in different parts of fish in Changsha market
|
|
quantity(tail) |
Header containing the gills |
Body cavity and gastrointestinal department |
trunk |
tail |
|
ribbonfish |
15 |
2 |
13 |
0 |
0 |
|
saury |
20 |
2 |
8 |
0 |
0 |
|
small yellow croaker |
30 |
0 |
0 |
0 |
0 |
|
turbot |
5 |
0 |
0 |
0 |
0 |
|
cuttlefish |
10 |
0 |
0 |
0 |
0 |
|
yellow teeth |
12 |
0 |
0 |
0 |
0 |
|
bummalo |
20 |
0 |
22 |
0 |
0 |
|
sardines |
30 |
0 |
0 |
0 |
0 |
3.4 Molecular identification
Anisakis larvaes were extracted from the fixative. Their whole genome DNA was extracted and its rDNA ITS region was amplified. Fragments amplified were consistent with the expected fragment, about 1 000 bp, as shown in the figure 3. It indicates that several common fish parasites in the Changsha market are mostly Anisakis.
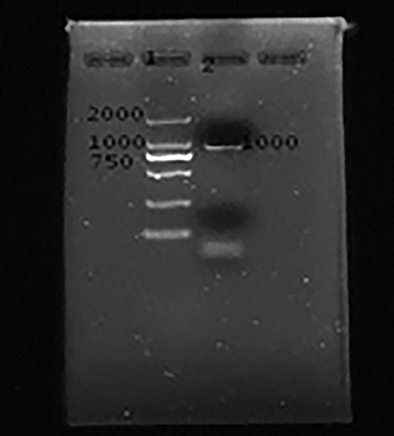
Figure 3. Electrophoresis gel imaging of PCR amplification products of the ITS region of the genus Anisakis. Lane1: PCR marker: DL2000 ,lane 2 :PCR amplification products in ITS region of Anisakis genera.
The total detection rate of the third-stage larvae of the genus Anisakis in the common fish in the Changsha market in this survey was 25%. Among the fishes sampled, except for the multi-treasure fish, the cuttlefish, the yellow three teeth, and the sardines, the detection rate was 0, and the detection rate of the other species was 100%. This result is different from the description of the master's thesis “Molecular identification and population genetic structure analysis of the parasitic nematodes in the East China Sea”, which is guided by Professor Zhang luping of Hebei Normal University[8]. It was pointed that the distribution of the genus Anisakis was global, and their adults and larvae located in domain ocean of the world, focusing mainly on the islands in the Pacific Ocean and the north Atlantic ocean and the Atlantic coast, in the majority with the Pacific Ocean[1, 7]. Along with the changes in the distribution of Marine fish, the genus Anisakis showed the seasonal distribution[9]. It is reported that hundreds of species of fish were infected with the genus Anisakis in more than twenty countries[10]. The highest infection rate was above 80%, respectively for herring, cod, rock fish. According to statistics, the genus Anisakis in fish was up to hundreds of species, in addition to molluscs, marine mammals. They have higher infectivity in mackerel, spanish mackerel, octopus, big fish, small fish, mackerel, cod, herring, Hyman and so on[11]. And the genus Anisakis showed no host specificity. Compared with the result reported by Chen Junhua in 2014,the positive rate of fish infected Anisakis larvae was slightly low and the range was slightly narrower. My point of view , further investigation is needed to determine whether it is related to the number of fish species sampled, the sampling season, the age of the fish, the distribution of fish stocks.
The nematodes collected in this time are more in the saury, octopus, small yellow croaker, and faucet fish, and are the dominant hosts of the fish parasitic nematodes commonly found in the Changsha market. Especially bummalo, more than half of fish acquired were found the infection of Anisakis. Although it shows no infection was found in four kinds of fish, such as turbot, cuttlefish, yellow teeth and sardines. But the result didn’t represent no infection among all fish because of a small number of acquisitions. After comparing and analyzing all the data, it was found that the fishes sampled were all frozen and transported by the sea, and part of their gastrointestinal tract had been decayed after thawing, which was also one reason for the low detection rate. In addition, there are two reasons. First of all, the source of fish on the market affects the infection rate of the Anisakis . The fish caught in the deep sea and coastal areas are transported after being frozen, and the probability of detecting the heterosexual worm is different from that of the farmed fish. The latter underwent drug intervention, and the infection rate of parasites in the same fish was lower than that of the former. Secondly ,the uncertain time capturing marine fish is also one of the important reason. The distribution of the genus Anisakis in the Pacific northwest and the northeastern Taiwan was surveyed by Chou and so on from April 2004 to March 2005. It showed there was the infection almost every month in this twelve-month period, the highest in April, followed in May, relatively least during the other months. From 178 cases of patients with Anisakis, surveyed by Fujino Longbo and so on, it was found that the highest incidence rate was from February to May, and there was a trend of decrease from June to August.
In this research, Anisakis larvae was not detected in the muscle of the trunk and tail but detected more in the body cavity, internal organs, gills, mesenteric and other parts especially after a long time of freezing. This suggest that we should slaughter and empty the fish internal organs immediately after cleaning. Try to prevent the fish from being contaminated during dissection, otherwise there is a danger of infection with Anisakis[12]. In Changsha, the residents have a very heavy taste , like to eat a variety of fresh foods, especially like to eat visceral hot pot and directly-soaked animal meat by wine , resulting in a greater chance of infection The study found that under the conditions of -2 ° C and -8 ° C for 96 hours, the Anisakis still have the ability to invade the rat tissue, and the ability disappears after 14 hours at -20 ° C. [13]. In Japan, there had been twelve cases infected Anisakis larvae due to eating raw genitalia of Squid[14].Anisakis larvae which was poor resistance to high and low temperature and it could be eliminated after heating at 60 ℃ high temperature for 10 minutes[15]. So, eating cooked fish was the most effective way to prevent Anisakiasis, and we should separate raw food from cooked food in order to avoid cross-infection. In addition, parasites would be guaranteed to be killed in the marine fishes frozen at - 20℃ temperature for 24 hours or at 4 ℃ temperature more than 5 days.
From 2005 to 2006, Du Chunxia adopted the molecular biology method to identify the Anisakis larvae and Hysterothylacium aduncumin in the Yellow Sea’s fish[16]. D’Amelio and so on used larvae fiber endoscopy sample was subjected to molecular identification of PCR-RFLP. The results not only proved this patient was infected with Anisakis, but also proved PCR-RFLP was a cost-effective and reliable identification tool of Anisakis larvae[17]. In this investigation, molecular biology (PCR) technology was only used to amplify for Anisakis among common fish for sale in changsha, universal primers of the genus Anisakis was used to amplify, and the amplification products were sequenced and compared by database. These results simply proved that detected nematode was the genera Anisakis, but not specific species identification.
The next work is mainly focused on the following areas: increase the number of the surveyed sample, track the source of the sample and classify the deep-sea fish and freshwater fish, classify different kinds of fish by body length and weight, carry on the statistical analysis on the infection status, PCR-RFLP molecular identification of the detected samples of Anisakis larvae, compare the sequencing results with existing sequences on Genebank, type for the detected Anisakis and remind the general public to pay attention to food hygiene by eating fish, reduce the incidence of Anisakis disease.
In conclusion, we based on randomly collected , morphological identification , and molecular biology (PCR) technology indicated the infection rate of the third stage larvae (L3) of Anisakis in marine fishes for sale in Changsha City, Human province, China . The result is the infection rate of the L3 of Anisakis in fish in Changsha market was extremely serious, and there was a certain relationship between the fish species and the site of infection. This study gave the general public a wake-up call and enhanced health and safety awareness. The limitation of this study is the samples surveyed were small and the same kind of fish was not classified by capture time, fish length, weight classification in this study, so the result was not statistically significant. However, this result of is worthy of recognition because it had not been investigated into marine fishes for sale in Changsha city so far. This study filled a gap in this area, gave the general public a wake-up call and enhanced health and safety awareness.
ZRM and YHH are equal contribution to the works.
JZ and XW are equal contribution to the works.
Shamsi S et al: Occurrence of anisakid parasites in marine fishes and whales off New Caledonia. Parasitology Research 2018, 117(10):3195-3204. PMid:30051335
View Article PubMed/NCBIBarcala E et al: Occurrence of Anisakis and Hysterothylacium larvae in commercial fish from Balearic Sea (Western Mediterranean Sea). Parasitology Research 2018, 117(12):4003-4012. PMid:30327920
View Article PubMed/NCBIGoffredo E et al: Prevalence of anisakid parasites in fish collected from Apulia region (Italy) and quantification of nematode larvae in flesh. International Journal of Food Microbiology 2019, 292:159-170. PMid:30599456
View Article PubMed/NCBIZhang L et al: The specific identification of anisakid larvae from fishes from the Yellow Sea, China, using mutation scanning-coupled sequence analysis of nuclear ribosomal DNA. Molecular and Cellular Probes 2007, 21(5-6):386-390. PMid:17604951
View Article PubMed/NCBIBaeza ML et al: Anisakis simplex allergy: a murine model of anaphylaxis induced by parasitic proteins displays a mixed Th1/Th2 pattern. Clinical and Experimental Immunology 2005, 142(3):433-440. PMid:16297154
View Article PubMed/NCBICaramello P et al: Intestinal localization of anisakiasis manifested as acute abdomen. Clinical Microbiology and Infection 2003, 9(7):734-737. PMid:12925120
View Article PubMed/NCBIGuardone L et al: Anisakis spp. larvae in different kinds of ready to eat products made of anchovies (Engraulis encrasicolus) sold in Italian supermarkets. International Journal of Food Microbiology 2018, 268:10-18. PMid:29306733
View Article PubMed/NCBIMolina-Fernandez D et al: Molecular epidemiology of Anisakis spp. in blue whiting Micromesistius poutassou in eastern waters of Spain, western Mediterranean Sea. International Journal of Food Microbiology 2018, 282:49-56. PMid:29902783
View Article PubMed/NCBICho TH et al: The time course of biological and immunochemical allergy states induced by anisakis simplex larvae in rats. Clinical and Experimental Immunology 2006, 143(2):203-208. PMid:16412043
View Article PubMed/NCBIKim JH et al: Comparative transcriptome analyses of the third and fourth stage larvae of Anisakis simplex (Nematoda: Anisakidae). Molecular and Biochemical Parasitology 2018, 226:24-33. PMid:30455159
View Article PubMed/NCBILanfranchi AL et al: Influence of confluent marine currents in an ecotonal region of the South-West Atlantic on the distribution of larval anisakids (Nematoda: Anisakidae). Parasites & Vectors 2018, 11(1). PMid:30409156
View Article PubMed/NCBIUna-Gorospe M et al: Occupational disease due to Anisakis simplex in fish handlers. International Maritime Health 2018, 69(4):264-269. PMid:30589066
View Article PubMed/NCBIDaschner A et al: Towards a differential definition of atopy:Anisakis simplexand the relationship between parasites and arthropods in respiratory allergy. Parasite Immunology 2008, 30(8):417-424. PMid:18507783
View Article PubMed/NCBIGaglio G et al: Anisakis spp. larvae in three mesopelagic and bathypelagic fish species of the central Mediterranean Sea. Parasitology International 2018, 67(1):23-28. PMid:28965943
View Article PubMed/NCBIShikino K, Ikusaka M: Anaphylaxis Induced by Anisakis. Internal Medicine 2019. PMid:30918192
View Article PubMed/NCBIZhu XQ et al: Identification of anisakid nematodes with zoonotic potential from Europe and China by single-strand conformation polymorphism analysis of nuclear ribosomal DNA. Parasitology Research 2007, 101(6):1703-1707. PMid:17694403
View Article PubMed/NCBIJung SK et al: Purification and Cloning of an Apoptosis-Inducing Protein Derived from Fish Infected with Anisakis simplex, a Causative Nematode of Human Anisakiasis. The Journal of Immunology 2000, 165(3):1491-1497. PMid:10903755
View Article PubMed/NCBI