Xiaoyu LUO
Email: xiaoyu.luo@mail.mcgill.ca
© 2019 Sift Desk Journals. All Rights Reserved
VOLUME: 4 ISSUE: 9
Page No: 970-985
Xiaoyu LUO
Email: xiaoyu.luo@mail.mcgill.ca
Xiaoyu LUO*, Jacqueline SEDMAN, Ashraf A. ISMAIL
Department of Food Science and Agricultural Chemistry, Faculty of Agricultural and Environmental Sciences, McGill University, 21111 Lakeshore Road, Sainte-Anne-de-Bellevue, QC Canada, H9X 3V9
Fazlurrahman Khan(fkhan055@pknu.ac.kr)
Jian Wang(jian.wang@etu.univ-lyon1.fr)
Ali Rafe(a.rafe@rifst.ac.ir)
Rui Li(liruihn@163.com)
Xiaoyu Luo, Jacqueline Sedman, Ashraf A. Ismail Microencapsulation of oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) essential oils in β-lactoglobulin(2019) Journal of Food Science & Technology 4(9)p:970-985
This study aimed to investigate the microencapsulation of oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) essential oils by emulsification/freeze-drying, using β-lactoglobulin (β-lg) as the coating material. Among the different essential oils, the freeze-dried capsules containing oregano essential oil showed a lower hygroscopicity. Dried powders with higher initial protein concentration exhibited higher bulk tapped density and particle density. The porosity of essential oil-embedded microcapsules ranged from 75.04% to 84.62%, with no significant difference in between different essential oil species (P > 0.05). The overall encapsulation efficiency of essential oils was determined to be in the range of 29.50 – 36.31%. All freeze-dried samples containing essential oils showed significant antioxidant activities in a β-carotene assay, with the percentage of inhibition ranging from 41.05% to 51.02%. The release kinetics of rosemary and sage essential oils from dried microcapsules into phosphate buffer solution were similar, whereas the release of oregano essential oil was considerably lower. FTIR analysis illustrated significant impact of oregano essential oil on the structure change of β-lg.
Key words: microencapsulation, essential oil, freeze-drying, β-lactoglobulin
In the last decades, there are remarkably rising demands for bioactive compounds, which usually referred to as nutraceuticals, encompassing antimicrobials, antioxidants, prebiotics, fatty acids etc. [1-6] These compounds are found to possess significant health benefits against certain diseases such as cancers, cardiovascular malfunction, type 2 diabetes, and autoimmune diseases [3-6]. However, as most bioactive compounds are easily degraded, research on novel food processing technology is continuously seeking for means to protect or incorporate these compounds into stable food matrices [7].
Among the many types of functional foods, one group that becomes increasingly popular is essential oils, which are hydrophobic liquid extracts from raw plants such as rosemary, thyme, clove, lavender, eucyptus, jasmine, orange etc. [8]. The significance of essential oils in food industry applications is attributed to their antimicrobial, antioxidant and antiviral characteristics [9,10]. Overall there are more than 3,000 known essential oil species, in which oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) essential oils are popular ones and thus selected for this study. In general, essential oils are being used commercially as food additives, sanitizers and natural remedies, in the fields of agriculture and medicine, as well as flavor and fragrance industry [10,11]. Most essential oils are composed of hundreds of chemicals, but it is generally accepted that the most abundance substances are terpenes and polyphenols [12]. In particular, the major components are carvacrol and thymol for oregano essential oil [13,14], 1,8-cineole and α-pinene for rosemary essential oil [15,16], and α-thujone and camphor for sage essential oil [17,18]. Due to the presence of these bioactive compounds, these essential oils are considered to possess biological functions on plants and human systems. The terpenoids and phenolic compounds can help protecting plants from pathogens and predators, while the active functions in the human body are diverse, by possessing antimicrobial, antioxidant, anti-inflammatory, anticancer, analgesic and sedative activities [10, 11].
As many essential oils are chemically unstable and susceptible to environmental degradations, certain protective techniques have to be applied to counteract this problem. Microencapsulation is an advanced technology that protects sensitive compounds from environmental factors by using a stable coating material and maintains significant biological and physicochemical characteristics of the core material [19]. One of the most common microencapsulation techniques is drying, which mainly involves spray-drying and freeze-drying [20]. Freeze-drying, also known as lyophilisation, is a process of freezing the water in the food followed by subliming the ice under vacuum. Because freeze-drying is operated at low temperature condition, heat-labile materials are suitable to be encapsulated by freeze-drying [21]. Moreover, this technique has been reported to have a better retention of volatile compounds, with excellent product quality [22,23]. However, freeze-drying has not been as extensively studied as spray-drying for the microencapsulation of bioactive compounds.
One crucial step in microencapsulation is the selection of a suitable coating material, which is usually a film-forming biopolymer that possesses emulsifying capacity [24,25]. The coating material is considered to protect the core active components from loss and damage during processing, transport and storage, as well as to provide convenience for controlled release and delivery. Among the many biopolymers, whey protein exhibits excellent emulsifying properties and has been used previously for microencapsulation of volatile compounds [26,27]. Since whey protein is a food waste in cheese industry, developing its usage in microencapsulation helps reduce food waste and contributes to sustainable agriculture. Being the major fraction in whey protein, β-lactoglobulin (β-lg) has been extensively studied for its great potential for encapsulating bioactive compounds such as β-carotene [28], riboflavin [29], caffeine [30] and catechin [31].
This study aimed to evaluate the microencapsulation of oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) essential oils in β-lg by freeze-drying. The physical properties, encapsulation efficiency and antioxidant activities of the microencapsulated particles were determined. The kinetics of controlled release of essential oils from the microcapsules was studied. The influence of essential oils on proteins secondary structure was investigated by Fourier transform infrared spectroscopy.
2.1 Materials
Pure oregano (Origanum vulgare L.), rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.) essential oils were purchased from a local grocery store in Montréal, Canada, and used without further purification. Food-grade bovine β-lactoglobulin (80% purity from bovine whey protein concentrate) was supplied by BioPure Products, Bellevue, WA USA. All the chemicals (sodium chloride, potassium chloride, potassium phosphate, sodium phosphate dibasic dehydrate and β-carotene) and organic solvents (toluene, n-hexane and chloroform) were from Sigma-Aldrich, Oakville, ON Canada.
2.2 Sample preparation and freeze-drying
Protein stock solutions (10%, 15% and 20% w/v) were prepared by adding β-lg powder (10 g, 15 g and 20 g) directly into a beaker containing 100 mL of distilled deionized water with constant stirring. The solutions were covered and left overnight for more than 20 hours to ensure β-lg was fully hydrated. For each treatment, the essential oil (0%, 5%, 10%, 15% and 20% w/w protein weight basis) was added to the prepared solution followed by immediate mechanical homogenization for 3 min with the speed at 8000 rpm, by employing a Polytron homogenizer (Westbury, NY USA). Each homogenized solution was subject to a filtration step through a Whatman No.1 filter paper, and stored in the freezer (-18 oC) for approximately 48 hours before freeze-drying.
The frozen samples were dried via a Labconco freeze dryer (Labconco Corporation, Kansas City, MO USA), by setting the freezer temperature at -50 oC and internal pressure lower than 25 mmHg. The dried powder was collected after 48 hours of drying and immediately transferred and stored in an airtight container at refrigerated temperature (4 oC). No further processing steps were implemented before subsequent analyses on the dried microcapsules.
2.3 Physical properties
The hygroscopicity of the dried powder was determined based on the method by Cai & Corke [32], with minor modifications. Specifically, for each treatment, approximately 1 g of the pre-weighed sample was placed in a dessicator containing saturated NaCl solution (75.29% RH) at 25 oC. All samples were reweighed after a 2-week storage period and the hygroscopicity was expressed as grams of adsorbed moisture per 100 g of dried powder.
Bulk tapped density (ρb) was tested as described in the studies by Goula & Adamopoulus [33] and Jafari et al. [34], with modifications. Approximately 0.5 g of dried powder was transferred into a 10-mL glass graduated cylinder, manually tapped and mixed by a mechanical mixer, until no apparent volume change at a vertical distance could be notified. The bulk tapped density was calculated based on the measured mass and volume of each sample.
The particle density of the samples was measured by pycnometry method [35]. For each replicate, 0.5 g of freeze-dried powder was weighed into a 10-mL pycnometer which was then filled with toluene. The particle density (ρp) was calculated based on:

where the volume of toluene can be obtained according to its density (0.867 g mL-1).
Porosity (εb) was a derived parameter from bulk tapped density and particle density of the sample, as shown in Equation (2):

2.4 Encapsulation efficiency
The encapsulation efficiency of essential oils was determined by direct solvent extraction method, using n-hexane as the solvent [36-40]. For each replicate, 1 g of freeze-dried powder was weighed into a centrifugation tube, which was then filled with 10 mL of n-hexane. The mixture was vortexed for 3 min followed by centrifugation at 5000 rpm for 5 min. After centrifugation, the supernatant was filtered through a Whatman No. 1 filter paper and transferred into a pre-weighed glass tube. The residual weight was considered as the amount of unencapsulated essential oil, i.e. surface oil. By neglecting the essential oil loss during the drying process, the encapsulation efficiency was expressed as:
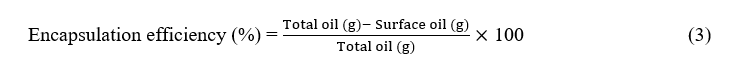
where the total oil refers to the essential oils added in the encapsulation process, and surface oil is the weight of essential oils extracted by n-hexane.
2.5 Antioxidant activity
The antioxidant activity of the freeze-dried capsules was determined by modified β-bleaching method [41-43]. Into a round-bottom flask, 6 mg of β-carotene, 60 μL of linoleic acid and 600 mg of Tween 60 were added together with boiling chips, followed by the addition of 30 mL of chloroform to dissolve all the chemicals. The chloroform was then removed by a rotary evaporator heated by a water bath at 50 oC. Oxygenated distilled water (150 mL) was added to form the reaction solution. For each treatment, 0.1 g of freeze-dried powder and 5 mL of the prepared solution were added into a small test tube, and immediately mixed for 1 min. For the control group, nothing was added except for the reaction mixture. All the prepared samples were tested for absorbance at 470 nm by a UV-Vis spectrophotometer (Thermo Scientific Genesys 10S, Thermo Fisher Scientific Inc., Waltham, MA USA), at 15-min intervals for 120 min. The percentage inhibition was calculated based on:
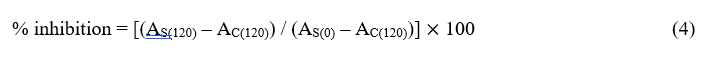
where AS(120) is the absorbance of the essential oil sample at 120 min, AC(120) is the absorbance of the control at 120 min, and AS(0) is the absorbance of the essential oil sample at 0 min. The freeze-dried samples containing 20% initial protein content and 15% (protein weight basis) essential oil were tested for antioxidant activity, in duplicates.
2.6 In vitro controlled release
The release profile of essential oils from freeze-dried powder was studied according to the dialysis method by previous studies [44-47]. For each replicate, approximately 5 g of dried powder was weighed into a dialysis bag with a molecular weight cut-off 6 – 8 kDa. The bag was then placed into a beaker containing 100 mL of pH 6.8 phosphate butter solution (PBS), with constant stirring. At regular time intervals, 1 mL of the solution was obtained and measured for UV absorbance (Thermo Scientific Genesys 10S), with maximum absorption wavelength values at 277 nm, 254 nm, 300 nm for oregano, rosemary and sage essential oils respectively, based on their respective predominant components. The freeze-dried samples containing 20% initial protein content and 15% (protein weight basis) essential oil were examined, in duplicates.
By assuming essential oils migrate from the microcapsules into the PBS solution by free diffusion, the following relationship [47,48] was adapted to construct the mathematical model of kinetics of controlled release:
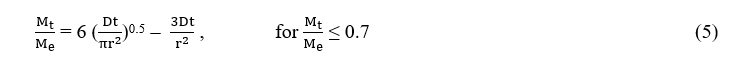
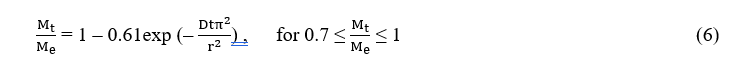
where Me is the weight of the essential oil released at equilibrium, Mt is the weight of the essential oil released at time t, D is the diffusion coefficient and r is the radius of the particle.
2.7 Fourier transform infrared spectroscopy analysis
Attenuated total reflectance – Fourier transform infrared (ATR – FTIR) spectroscopy was conducted on all pure samples, and freeze-dried capsules with different essential oil loadings (0%, 5%, 10%, 15% and 20% on protein weight basis). The infrared spectra of samples were recorded in the region of 4000 – 600 cm-1, with a resolution of 4 cm-1. For each same sample, an average spectrum of 128 co-added scans were recorded in triplicates, subsequently averaged by OMNIC 7.0 software. Resolution of the spectra was enhanced by using Fourier self-deconvolution (FSD) with a bandwidth of 44.4 and enhancement of 3.0. In order to study protein structural changes with presence of different essential oils, the region of the protein amide I band (1700 – 1600 cm-1) in the FSD spectra was normalized and the area of each amide I band component was determined with baseline correction. The area under each normalized IR band in the amide I region was calculated and resolved to match the corresponding secondary structures of β-lg.
2.8 Statistical analysis
The statistical significance of the physical properties of the microcapsules, including hygroscopicity, bulk tapped density, particle density, porosity and encapsulation efficiency, was assessed by analysis of variance (ANOVA) followed by Tukey HSD with 95% confidence interval, in Microsoft Excel (Redmond, WA USA). For the study on in vitro controlled release of essential oils in phosphate buffer solution, nonlinear parameter estimation was performed in Microsoft Excel (Redmond, WA USA), to fit the experimental data into the model, as shown in Equations (5) and (6).
3.1 Hygroscopicity
Moisture is an important factor affecting the shelf life and stability of microencapsulated particles since the absorption of water will lead to the loss of bioactive compounds and influence the rates of lipid oxidation. Hygroscopicity is a measure of water uptake by a sample from the surroundings over a period of storage. The hygroscopicity values of the freeze-dried protein powders containing different essential oils are shown in Fig. 1. It can be seen that the hygroscopicity of the microcapsules slightly increased with higher initial β-lg concentrations. This may be explained by the hygroscopic nature of β-lg. The hygroscopicity results for rosemary and sage essential oils were similar, ranging from 16.06% to 18.11%. Similar values were reported in a previous study on microencapsulation of rosemary oil by spray-drying using gum Arabic, ranging between 15.87% and 18.90% [49]. However, the values in Fig. 1 for microcapsules containing oregano oil (8.38 to 11.88%) are lower than those for the other essential oil samples. This may be due to the interactions between the essential oil and protein molecules, which reduced the possibility for proteins to bind with water.
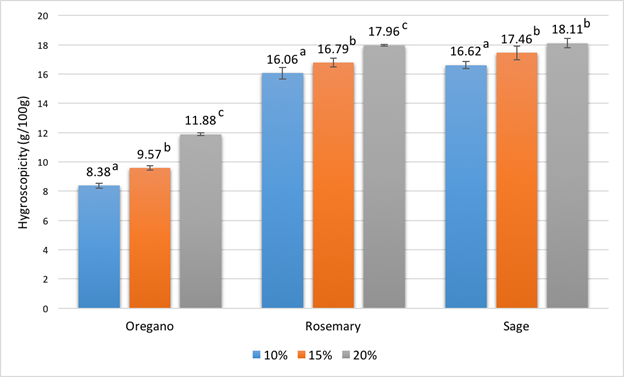
Fig. 1 Hygroscopicity of freeze-dried protein microcapsules containing different essential oils. The initial protein concentrations are indicated by different colors of the bars. For each type of essential oil, values with different letters are significantly different (P < 0.05).
3.2 Bulk Tapped Density, Particle Density and Porosity
In the food processing industry, bulk tapped density is an essential quality parameter in relation to packaging, distribution and storage of food powders since it reveals how much of the powder can be filled into a container with fixed volume [50]. Compared to bulk density, tapped density is a better indicator of the actual compressibility of a dry product. It is known that food powders with higher tapped density can be stored in smaller containers [51]. It can be seen from Table 1 that the bulk tapped density increased with higher initial protein concentrations (P < 0.05) for the dried powders with each essential oil as the core material. Comparison of the values for different essential oil products indicates that the bulk tapped density did not differ significantly between rosemary and sage essential oil samples, whereas the bulk tapped density for the microcapsules containing oregano essential oil was apparently higher. This difference revealed the higher compressibility of the dried powders containing oregano essential oil compared to those containing the other two types of essential oil. Overall, the bulk tapped density results for all samples ranges from 0.117 g cm-3 to 0.477 g cm-3, which are similar to the values reported in a previous study on microencapsulation of rosemary essential oil using whey protein and inulin as coating materials [35].
Particle density of a dry product is another key parameter since it influences the separation of different particles within a mixture [52]. Particle density is usually determined by a combination of factors such as the solid density, the internal space of the particles and the specific arrangement of particles if applicable [53]. The study by Shenoy et al. [54] suggested that particle density significantly influenced the mixture quality of binary mixes. In the present study, the trend for particle density was similar to that of bulk tapped density. The microcapsules produced with higher protein concentrations showed higher particle density values (P < 0.05), indicating that higher amounts of coating materials would result in a compression of the particles in the final product. In general, the particle density for all samples was in the range of 0.603 – 2.561 g cm-3, which was acceptable based on comparison with the value (1.55 g cm-3) obtained from a study on encapsulation of cardamom essential oil in mesquite gum [55]. The particle density values did not vary greatly among different essential oil samples at the same initial protein content level. However, the particle density of oregano essential oil microcapsules was slightly higher than the other two essential oil treatments, at the same protein concentration, indicating better compatibility of β-lg protein molecules with oregano essential oil. This finding may be attributed to significant intermolecular bindings in between the predominant species in oregano essential oil with certain regions of the protein structure.
Porosity is a derived value from tapped density and particle density of the dried powders. It is known that the porosity of milk-based powders is influenced by several factors such as drying methods, particle size and storage conditions [56]. The significance of porosity for powder products is that it controls the rehydration rate, thereby revealing the shelf-life stability [57]. The results in Table 1 show that for samples containing the same essential oil, initial protein concentration did not affect the porosity of the final products (P > 0.05). By comparing the values for encapsulation of different essential oils, it can be seen that the porosity for microcapsules containing oregano essential oil (66.68 – 83.36%) was slightly lower than the values for microcapsules containing rosemary (78.08 – 89.67%) and sage (79.66 – 87.86%) essential oils. This result indicates that the powders containing oregano essential oil as the core component would have a lower rehydration rate. The previous study on encapsulation of rosemary essential oil using a whey protein and inulin mixture as wall system obtained similar results (80.2 – 82.8%) for this physical property [35].
Table 1. Bulk tapped density, particle density and porosity of freeze-dried protein microcapsules containing essential oils
|
Essential oil |
Initial protein concentration (%) |
Bulk tapped density (g cm-3) |
Particle density (g cm-3) |
Porosity (%) |
|
Oregano |
10 |
0.222 ± 0.004a |
0.938 ± 0.199a |
75.36 ± 4.66a |
|
|
15 |
0.342 ± 0.010b |
1.484 ± 0.428ab |
75.04 ± 6.81a |
|
|
20 |
0.457 ± 0.017c |
2.045 ± 0.383b |
76.74 ± 4.93a |
|
Rosemary |
10 |
0.123 ± 0.005a |
0.847 ± 0.203a |
84.62 ± 3.60a |
|
|
15 |
0.166 ± 0.006b |
1.123 ± 0.252b |
84.22 ± 4.36a |
|
|
20 |
0.301 ± 0.006c |
1.790 ± 0.299c |
82.73 ± 2.82a |
|
Sage |
10 |
0.125 ± 0.003a |
0.701 ± 0.086a |
81.90 ± 1.89a |
|
|
15 |
0.198 ± 0.017b |
1.268 ± 0.278b |
83.75 ± 3.34a |
|
|
20 |
0.266 ± 0.007c |
1.453 ± 0.096bc |
81.64 ± 0.91a |
*Values are reported as mean ± standard deviation. For each type of essential oil, values with different letters in the same column are significantly different (P < 0.05).
3.3 Encapsulation efficiency
For encapsulation technology, the encapsulation efficiency is an essential index which directly reveals how much of the active compounds can be protected within the coating system. The results for this parameter obtained by direct solvent extraction are shown in Table 2.
Table 2. Encapsulation efficiency (%) of essential oils (EOs) in freeze-dried microcapsules determined by solvent extraction
|
Initial protein concentration (%) |
Encapsulation efficiency (%) |
||
|
Oregano EO |
Rosemary EO |
Sage EO |
|
|
10 |
29.50 ± 4.91a |
33.75 ± 1.94a |
30.53 ± 0.75a |
|
15 |
31.37 ± 2.14a |
35.56 ± 5.05a |
34.00 ± 1.62b |
|
20 |
36.31 ± 4.15a |
35.78 ± 3.75a |
34.76 ± 4.46ab |
*Values are reported as mean ± standard deviation. For each column, values with different letters are significantly different (P < 0.05).
The overall range for all treatments was 23.12 – 42.13%. It can be seen that the initial protein concentration did not affect the encapsulation efficiency for most samples (P > 0.05), as long as the ratio of the amount of essential oil to protein was fixed. Using the same method, the encapsulation efficiency of the microencapsulation of flax oil in zein protein ranged from 32.68% to 59.63% [51], while the results for encapsulating wheat germ oil in whey protein and maltodextrin mixture were between 51.29% and 89.62% [40]. Another study on encapsulation of rosemary essential oil by freeze-drying using whey protein and maltodextrin as the wall materials [58] employed Soxhlet extraction to determine the encapsulation efficiency and obtained much higher values (69.90 – 95.54%). In general, the encapsulation efficiency of a microencapsulation process is usually influenced by multiple factors involving the identity of the wall materials, the concentration or relative ratio of each component, the encapsulation method, and the method used to determine encapsulation efficiency, among others. The relatively low encapsulation efficiency results obtained in this study by comparison with the other studies mentioned above can be explained by differences in wall system selection and extraction methods. A mixture of coating materials will further increase the encapsulation efficiency by synergistic effects. For instance, a study using whey protein and maltodextrin as the coating materials showed reduced droplet sizes in encapsulating orange oil, due to better solubility compared to using whey protein alone [59]. The importance of carbohydrates in wall systems is attributed to their enhancement of the drying properties of the final products [60]. The presence of whey protein was important due to its excellent emulsifying capacity. Previous studies have indicated that a higher amount of whey proteins would result in higher encapsulation efficiency due to the reduction in viscosity of the emulsion induced by protein structural changes [34]. To improve the encapsulation efficiency, more studies will be conducted in the future to investigate the particle size of the protein matrix, which plays an important role in influencing the movement of essential oils into the porous macromolecules. Another key factor, the morphology of the microcapsules will be visualized by microscopic imaging techniques in future work.
Determination of encapsulation efficiency by solvent extraction and Soxhlet extraction may also result in different results. In this study, preliminary tests using Soxhlet extraction did not efficiently extract the surface oils from the microcapsules, and hence direct solvent extraction was employed. In addition, it should be noted that for the calculation of the encapsulation efficiency using Equation (3), the loss of essential oil during freeze-drying was assumed to be negligible, and therefore the oil retention in the freeze-dried powders was assumed to be 100%. The common method used for determining total oil retention in the literature is hydrodistillation in a Clevenger-type apparatus [58,61]. In a study on encapsulation of rosemary essential oil in a whey protein and maltodextrin mixture (3:1), the total oil retention after freeze drying ranged from 68.68% to 94.80% [58]. Thus, in extending the present studies, experimental determination of oil retention during freeze drying will be an important consideration for the development of essential oil encapsulations in different wall systems.
3.4 Antioxidant activity
Determination of antioxidant activity by β-carotene assay was based on the reaction between β-carotene and the radicals produced by linoleic acid oxidation, which results in gradual loss of the orange/yellow color [62]. Thus, this method actually measures the ability of antioxidants to slow down the color degradation by scavenging the free radicals [12]. For samples containing different essential oils, the average rate of β-carotene bleaching over 2 hours is shown in Fig. 2.
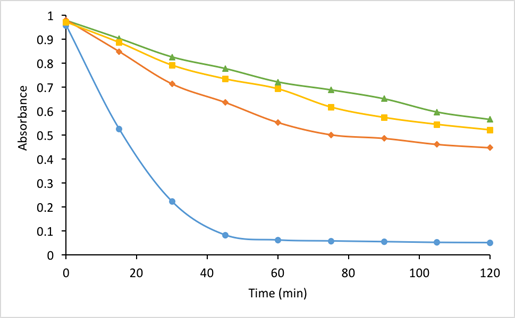
Fig. 2 Oxidation rate of β-carotene assay over time for the treatment with microcapsules containing oregano (◆), rosemary (■) and sage (▲) essential oils, compared to control (●).
From Fig. 2, it can be seen that all microcapsules containing different essential oils showed significantly reduced oxidation rates, compared to the control group. Although the absorbance for all samples decreased gradually, the results demonstrated effectiveness of antioxidant activity of freeze-dried capsules containing all three types of essential oil. The overall inhibition on β-carotene oxidation was differed slightly for the powers containing different essential oils. The dried products with oregano essential oil showed a lower percent inhibition (42.86%) than those with the other two essential oils, 51.06% and 55.50% for rosemary and sage essential oils respectively (P < 0.05). This may be explained by multiple reasons. First, the various chemical compositions of different essential oils are fundamental factors that affect their antioxidant activity. The study by Kulisic et al. [42] on evaluation of oregano essential oil obtained more than 50% inhibition using β-carotene bleaching assay. Using the same method, another study on the essential oil of Hymenocrater longiflorus Benth. found the antioxidant activity to be 54.6%, 50.0% and 64.7%, for total phenols, polar fraction and non-polar fraction, respectively [63]. The major chemical compounds in the essential oil of Hymenocrater longiflorus Benth. include α-pinene, 1,8-cineole, β-eudesmol, and spathulenol, some of which are also present in rosemary and sage essential oils [18,63,64]. Second, since microcapsules were tested instead of pure essential oils, the difference in powder solubility is another factor that may have had an impact on the results. In addition, the possible difference in the amount of available essential oil per unit weight of microcapsules should be also taken into consideration. Previous studies also demonstrated antioxidant activity of essential oil extracts or in the form of microcapsules by other methods such as oxygen radical absorbance capacity assay [44], conjugated diene assay [65], 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [66], thiobarbituric acid reactive species (TBARS) test [67] and ferric reducing/antioxidant power (FRAP) assay [68]. These methods will give different results in terms of antioxidant activities due to different reactants and detection techniques. Therefore, for further studies, multiple techniques can be applied to evaluate the antioxidant activities of microcapsules containing different essential oils.
3.5 In vitro controlled release
In addition to protecting bioactive compounds from degradation, controlled release into specific environments is one of the purposes of microencapsulation technology in the food industry. In general, the movement of microencapsulated essential oils to the exterior of the micropores is usually achieved by their diffusion via the polymer matrix [69]. The diffusion rate often declines gradually over time, and thus it is of value to study the kinetics in order to evaluate the efficacy of a polymeric matrix for releasing essential oils [70]. Among the many factors that may affect the diffusion process from the same matrix, the vapor pressure is considered to be the most important driving force [71,72]. In the present study, the kinetics of controlled release for the microcapsules containing different essential oils in PBS was evaluated and is shown in Fig. 3. Using Equations (5) and (6) based on Fickian diffusion, a mathematical model was generated by non-linear parameter estimation and is also shown in Fig. 3. The values of the sum of the squared deviations (SSD) indicate how close the model-generated data fit to the experimental results (the smaller the value, the better the fit). The comparison of the release profiles obtained for the three microencapsulated essential oils is shown in Fig. 4.
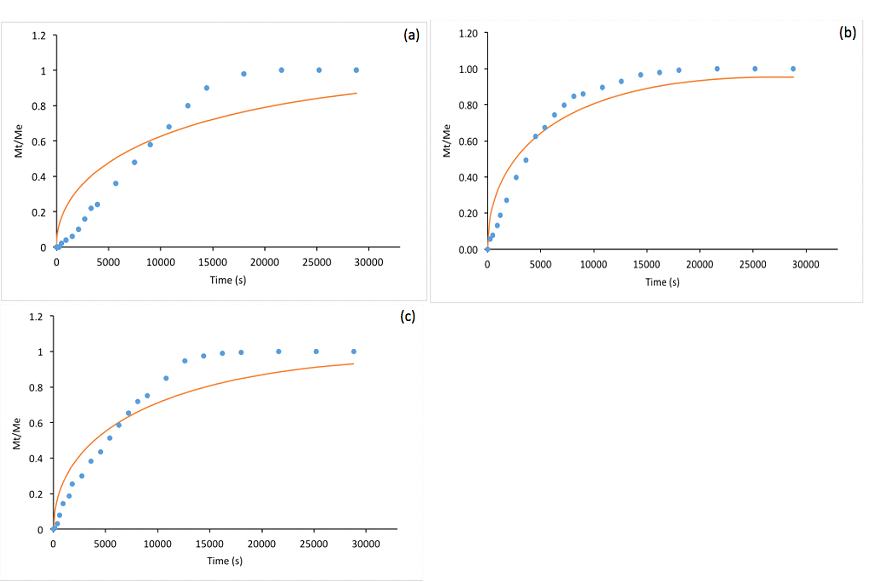
Fig. 3 Kinetics of release of oregano (a), rosemary (b), and sage (c) essential oils from freeze-dried β-lg microcapsules over 8 hours. Mt is the mass of the essential oil released at time t and Me is the mass of the essential oil released at equilibrium. Scatter plot = experimental results; smooth solid line = model-generated data. Sum of squared deviations = 0.49, 0.29 and 0.19 for oregano, rosemary and sage essential oils respectively.
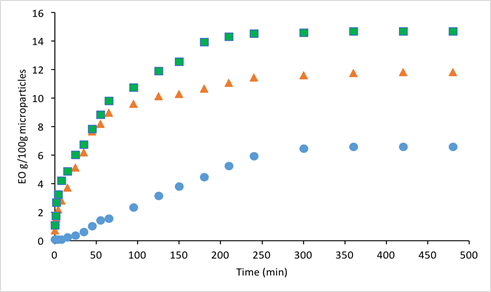
Fig. 4 Release profiles of freeze-dried microparticles containing different essential oils: oregano (●), rosemary (▲), sage (■) over 8-hour controlled release in PBS. EO= essential oil.
From Fig. 3, it can be seen that the release of all the essential oils can be fitted to the model-generated values, among which the samples containing rosemary essential oil showed the best fit with the lowest SSD value (0.19). Fig. 4 shows a common trend for all the samples that most of the essential oils were released during the first 3 hours; after that, the “lag” phase where the essential oil concentrations in PBS became equilibrium was reached. This general trend and the model-fitted data in Fig. 3 are consistent with the results obtained by another study on microparticles containing oregano essential oil using various types of polymeric matrices [44]. By comparing with different essential oils, it can be seen that the release of rosemary and sage essential oils from the protein matrix was significantly higher than that of oregano essential oil. This may be attributed to the differences in their vapor pressures. Based on literature information on the major components, the vapor pressures of each essential oil at room temperature (~25 oC) are approximately 0.0296 – 0.0376 mmHg for oregano [73], 1.90 mmHg for rosemary [74], and 1.90 mmHg for sage [75] essential oil, respectively. Based on this comparison, the vapor pressure of oregano essential oil is significantly lower than that of the other two essential oils, indicating a smaller driving force to initiate the diffusion process. It should be noted that other factors may also influence the diffusion process, such as the interactions between the core and coating materials, the size of the microparticles, and the homogeneity of the essential oils distributed in the micropores, as well as the consistency of the particle dimensions over time [71].
3.6 Fourier transform infrared spectroscopy (FTIR) analysis
In order to characterize the freeze-dried microcapsules by FTIR spectroscopy, the FTIR spectra of pure essential oil samples were first recorded and the assignments of the major IR bands associated with chemical components unique to each essential oil were identified. For oregano essential oil, the signature band for carvacrol was located at 811 cm-1, indicating the C-H out-of-plane bending [44]. The two strong bands (1745 and 984 cm-1) of rosemary essential oil, suggesting C=O stretching and C-H out-of-plane bending, demonstrate the presence of camphor and 1,8-cineol [77]. For sage essential oil, the carbonyl stretching band at 1745 cm-1 dictates the presence of α-thujone and camphor [76]. The bands in the region between 3000 and 2900 cm-1, assigned to C-H stretching vibrations, are common to all of these essential oils and thus are not listed in the table. FTIR spectra of freeze-dried β-lg samples containing each essential oil are presented in Fig. 5.
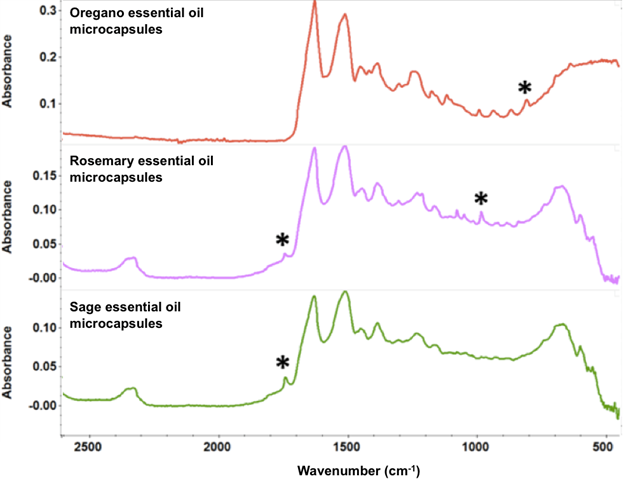
Fig. 5 Stacked ATR-FTIR spectra of freeze-dried b-lg samples containing essential oils at a concentration of 20%.
The two most intense bands in the spectra in Fig. 5 are the amide I and amide II bands of β-lg at 1628 and 1514 cm-1, respectively. The amide I band of proteins, which is due mostly to C=O stretching of peptide linkages, consists of overlapping bands of different protein secondary structure components such as α-helix, β-sheets, β-turns and random coils, which can be resolved by Fourier self-deconvolution or other resolution-enhancing methods [78]. Thus, in order to examine the interaction between essential oils and β-lg, analysis of the Fourier self-deconvoluted (FSD) amide I band in the spectra of the freeze-dried b-lg samples containing different concentrations of essential oils was conducted. The FSD amide I bands involves protein secondary structures at different wavenumber regions, where α-helix and β-turns are at 1654 and 1667 cm-1 respectively; β-sheets have two distinctive bands at 1629 and 1692 cm-1; random coils are located at 1642 cm-1 of the IR spectrum. Table 3 shows the percent contribution of each secondary-structure estimated from the relative areas of the component bands in the FSD spectrum. It should be noted that the absolute band intensities in these spectra cannot be compared because they are affected by the contact between the sample and the ATR surface, which is variable from sample to sample. On the other hand, the values in Table 3 reflect the relative intensities of the amide I component bands and hence can be interpreted in terms of changes in the relative proportions of the different secondary structure components.
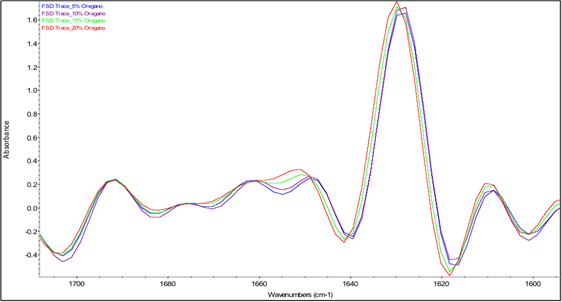
Fig. 6(a) Fourier self-deconvoluted protein amide I region (1700-1600 cm-1) of averaged IR spectra of β-lg containing oregano essential oil at different concentrations.
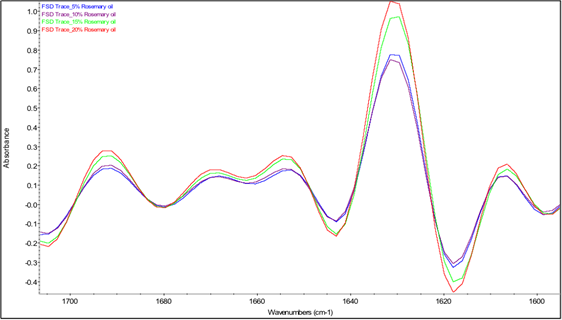
Fig. 6(b) Fourier self-deconvoluted protein amide I region (1700-1600 cm-1) of averaged IR spectra of β-lg containing rosemary essential oil at different concentrations.
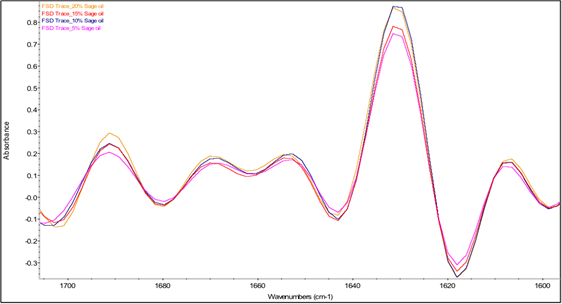
Fig. 6(c) Fourier self-deconvoluted protein amide I region (1700-1600 cm-1) of averaged IR spectra of β-lg containing sage essential oil at different concentrations.
Table 3. FTIR secondary-structure analysis of freeze-dried β-lg samples containing different essential oils (EOs)
|
Sample |
Percentage of secondary structure (%) |
||
|
α-Helix |
β-Turn |
β-Sheet |
|
|
β-lg (powder) |
11.7 |
3.9 |
84.4 |
|
5% Oregano EO |
7.5 |
5.4 |
87.0 |
|
10% Oregano EO |
7.4 |
5.3 |
87.2 |
|
15% Oregano EO |
6.9 |
2.6 |
90.4 |
|
20% Oregano EO |
9.5 |
1.2 |
89.3 |
|
5% Rosemary EO |
8.0 |
4.3 |
87.6 |
|
10% Rosemary EO |
8.4 |
4.5 |
87.2 |
|
15% Rosemary EO |
9.5 |
4.0 |
86.4 |
|
20% Rosemary EO |
9.3 |
4.1 |
86.6 |
|
5% Sage EO |
8.1 |
5.5 |
86.3 |
|
10% Sage EO |
8.7 |
6.0 |
85.3 |
|
15% Sage EO |
8.8 |
6.6 |
84.6 |
|
20% Sage EO |
7.6 |
7.0 |
85.5 |
Fig. 6 shows little effect of adding rosemary or sage essential oil into the protein matrix on the amide I band profile. In contrast, the spectra of the samples containing oregano essential oil show a different amide I band profile, which is dependent on the concentration of the essential oil. Specifically, in the spectra of the samples containing 5% and 10% oregano essential oil, all the bands with the exception of the band at 1692 cm-1 were slightly shifted to lower frequencies, and an additional band at 1675 cm-1 was observed. These observations suggest that the protein interacts strongly with components of oregano essential oil, resulting in perturbation of its secondary structure, similar to findings for drug-protein interactions from previous studies by Tajmir-Riahi [79]. However, as the concentration of oregano essential oil was increased to 15% and 20%, the bands progressively shifted back toward their original positions in the spectrum of β-lg and the intensity of the band at 1675 cm-1 is substantially diminished.
Additional information on the effects of the essential oils on the secondary structure of β-lg can be provided by examination of the FTIR estimates of the percentages of the secondary structure components in Table 3. Based on these estimates, the presence of oregano, rosemary and sage essential oils caused significant decrease in the percentage of α-helical structure and increase in the percentage of β-sheet structure. These results may indicate that the interaction of essential oil components with β-lg occurred at the surface of the protein between the α-helix and β-barrel, as reported in the literature for hydrophobic ligand binding with β-lg [80]. It may be noted that the lowest percentages of α-helical structure were obtained for samples containing oregano essential oil at concentrations of 5%, 10%, and 15% while the highest percentages of β-sheet structure were obtained for samples containing oregano essential oil at concentrations of 15% and 20%. The latter samples also exhibited a substantial decrease in the percentage of β-turn structure. Thus, both the band shifts discussed above and the estimated percentages of secondary structure components based on relative band intensities indicate that β-lg interacted more strongly with components of oregano essential oil than with the components of the other two essential oils employed in preparing freeze-dried microcapsules. These findings are consistent with the release profiles presented in Fig. 4, which showed that the release of oregano essential oil from the β-lg microcapsules was significantly lower than that of rosemary and sage essential oils. For future studies, the effects of varying the protein/essential oil ratio on the release profile should be investigated, as the FTIR studies presented here indicated that the nature of the interactions between β-lg and components of oregano essential oil depended on the concentration of the essential oil.
In conclusion, the microencapsulation of oregano, rosemary and sage essential oils in β-lg by freeze-drying was successfully performed in this study. The encapsulation efficiency was determined to be in the range of 29.50 – 36.31%. The microparticles containing oregano essential oil showed a lower hygroscopicity, higher bulk and particle density and lower porosity, compared to those containing rosemary essential oil and sage essential oil, revealing a lower rehydration rate and higher shelf-life stability. All dried powders containing the essential oils showed significant antioxidant activities via a β-carotene bleaching test. In a phosphate buffer solution, rosemary and sage essential oils were released readily and the amount released was much higher than that for oregano essential oil. FTIR analysis on protein conformational changes demonstrated there were significant interactions between essential oil components and β-lg, particularly in the case of oregano essential oil. Potential binding sites were proposed to be in the cleft on the surface of the protein between the α-helix and the β-barrel.
For future studies, the encapsulation process of essential oils can be further developed by improving the homogenization step or using a secondary coating material. The stability of essential oils in the microencapsulation process at different environmental conditions can be studied, such as varying temperature, pH and ionic strength of the solutions. The antimicrobial test of essential oils encapsulated in protein matrices should be conducted, in order to further demonstrate the efficacy against common food microorganisms. The controlled release study of essential oil microcapsules in organic solvents or in vivo study may be of interest, as well as the release study at other different pH conditions to simulate human’s digestive tract. The hydrophobic ligand binding between the major essential oil components with β-lg can be further investigated to determine the binding sites and binding affinities by other techniques such as fluorescence spectroscopy and isothermal titration calorimetry. Additionally, studying other essential oils for which there is less information available in the literature will also be of interest.
Mulat M, Pandita A, Khan F. Medicinal plant compounds for combating the multi-drug resistant pathogenic bacteria: a review. Curr Pharm Biotechnol. 2019;20(3):183-96. PMid:30854956
View Article PubMed/NCBIMulat M, Khan F, Muluneh G, Pandita A. Phytochemical profile and antimicrobial effects of different medicinal plant: current knowledge and future perspectives. Curr Tradit Med. 2020;6(1):24-42.
Fearon IM, Faux SP. Oxidative stress and cardiovascular disease: novel tools give (free) radical insight. J. Mol. Cell Cardiol. 2009;47:372-381. PMid:19481547
View Article PubMed/NCBIHuang M-T, Ghai G, Ho C-T. Inflammatory process and molecular targets for anti-inflammatory nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004;3:127-139.
View ArticleKaur C, Kapoor HC. Antioxidants in fruits and vegetables : the millennium's health. Int. J. Food Sci. Technol. 2001;36:703-725.
View ArticleLee J, Koo N, Min DB. Reactive oxygen species, aging and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004;3:21-33.
View ArticleKimpel F, Schmitt JJ. Review: Milk proteins as nanocarrier systems for hydrophobic nutraceuticals. J. Food Sci. 2015;80:2361-2366. PMid:26467442
View Article PubMed/NCBIRubiolo P, Sgorbini B, Liberto E, Cordero E, Bicchi C. Essential oils and volatiles: sample preparation and analysis: A review. Flavour Fragr. J. 2010;25:282-290.
View ArticlePourmortazavi SM, Hajimirsadeghi SS. Supercritical fluid extraction in plant essential and volatile oil analysis. J. Chromatogr. 2007;1163:2-24. PMid:17624357
View Article PubMed/NCBIValgimigli L. Essential oils as natural food additives: composition, applications, antioxidant and antimicrobial properties. New York, NY: Nova Science Publishers, 2012.
Baser KC, Buchbauer G. Handbook of Essential Oils: Science, Technology, and Applications. New York: CRC Press Taylor & Francis Group; 2010.
View ArticleMiguel MG. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules. 2010;15:9252-9287. PMid:21160452
View Article PubMed/NCBIGovaris A, Solomakos N, Pexara A, Chatzopoulou P. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol. 2010;137:175-180. PMid:20060188
View Article PubMed/NCBIHussain AI, Anwar F, Rasheed S, Nigam PS, Janneh O, Sarker SD. Composition, antioxidant and chemotherapeutic properties of the essential oils from two Origanum species growing in Pakistan. Rev. Bras. Farmacogn. 2011;21:943-952.
View ArticleJalali-Heravi M, Moazeni RS, Sereshti H. Analysis of Iranian rosemary essential oil: Application of gas chromatography-mass spectrometry combined with chemometrics. J. Chromatogr. A. 2011;1218:2569-2576. PMid:21429498
View Article PubMed/NCBIOrhan I, Aslan S, Kartal M, Sener B, Baser KHC. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. J. Food Chem. 2008;108:663-668. PMid:26059146
View Article PubMed/NCBILanga E, Della Porta G, Palavra AMF, Urieta JS, Mainar AM. Supercritical fluid extraction of Spanish sage essential oil: Optimization of the process parameters and modelling. J. Supercrit. Fluid. 2009;49:174-181.
View ArticleTaarit MB, Msaada K, Hosni K, Hammami M, Kchouk ME, Marzouk B. Plant growth, essential oil yield and composition of sage (Salvia officinalis L.) fruits cultivated under salt stress conditions. Ind. Crops Prod. 2009;30:333-337.
View ArticleBakry AM, Abbas S, Ali B, Majeed H, Abouelwafa, MY, Mousa A, Liang L. Microencapsulation of oils: A comprehensive review of benefits, techniques and applications. Compr. Rev. Food Sci. Food Saf. 2016;15:143-182.
View ArticleSilva KA, Coelho MA, Calado VMA, Rocha-Leao MHM. Olive oil and lemon salad dressing microencapsulated by freeze-drying. LWT-Food Sci. Tech. 2013;50:569-574.
View ArticleWilkowska A, Ambroziak W, Czyzowska A, Adamiec J. Effect of microencapsulation by spray-drying and freeze-drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol. J. Food Nutr. Sci. 2016;66:11-16.
View ArticleChranioti C, Karaberi A, Tsakanilka L-A, Tzia C. Freeze-dried fennel oleoresin products formed by biopolymers: Storage stability and characterization. Food Bioprocess Tech. 2016;9:2002-2011.
View ArticleKrokida MK, Philippopoulos C. Volatility of apples during air and freeze drying. J. Food Eng. 2006;73:135-141.
View ArticleJafari SM, Assadpoor E, He Y, Bhandari B. Encapsulation efficiency of food flavours and oils during spray drying. Dry. Technol. 2008;26:816-835.
View ArticleKim YD, Morr CV. Microencapsulation properties of gum Arabic and several food proteins: spray-dried orange oil emulsion properties. J. Agr. Food Chem. 1996;44:1314-1320.
View ArticleBernard C, Regnault S, Gendreau S, Charbonneau S, Relkin P. Enhancement of emulsifying properties of whey proteins by controlling spray-drying parameters. Food Hydrocolloids. 2011;25;758-763.
View ArticleRosenberg M, Lee SJ. Preparation and some properties of water-insoluble, whey protein-based microcapsules. J. Microencapsul. 2000;17:29-44. PMid:10670938
View Article PubMed/NCBIYi J, Lam TI, Yokoyama W, Cheng LW, Zhong F. Beta-carotene encapsulated in food protein nanoparticles reduces peroxyl radical oxidation in Caco-2 cells. Food Hydrocolloids. 2015;43:31-40.
View ArticleChen L, Subirade M. Effect of preparation conditions one the nutrient release properties of alginate-whey protein granular microspheres. Eur. J. Pharm. Biopharm. 2007;65:354-362. PMid:17150342
View Article PubMed/NCBIGunasekaran S, Xiao L, Ould Eleya MM. Whey protein concentrate hydrogels as bioactive carriers. J. Appl. Polym. Sci. 2006;99:2470-2476.
View ArticleZhang P, Dudhani A, Lundin L, Kosaraju SL. Macromolecular conjugate based particulates: preparation, characterisation and evaluation of controlled release properties. Eur. Polym. J. 2009;45:1960-1969.
View ArticleCai YZ, Corke H. Production and properties of spray-dried Amaranthus betacyanin pigments. J. Food Sci. 2000;65:1248-1252.
View ArticleGoula AM, Adamopoulus KG. Effect of maltodextrin addition during spray drying of tomato pulp in dehumidified air: II. Powder properties. Dry. Technol. 2008;26:726-737.
View ArticleJafari S-M, Mahdavi-Khazaei K, Hemmati-Kakhki A. Microencapsulation of saffron petal anthocyanins with cress seed gum compared with Arabic gum through freeze drying. Carbohydr. Polym. 2016;140:20-25. PMid:26876823
View Article PubMed/NCBIFernandes RVB, Borges SV, Botrel DA, Rodrigues C. Physical and chemical properties of encapsulated rosemary essential oil by spray drying using whey protein-inulin blends as carriers. Int. J. Food Sci. Technol. 2014;49:1522-1529.
View ArticleBylaite E, Venskutonis PR, Mazdzieriene R. Properties of caraway (Carum carvi L.) essential oil encapsulated into milk protein-based matrices. Eur. Food Res. Technol. 2001;212:661-670.
View ArticleHundre SY, Karthik P, Anandharamakrishnan C. Effect of whey protein isolate and β-cyclodextrin wall systems on stability of microencapsulated vanillin by spray-freeze drying method. Food Chem. 2015;174:16-24. PMid:25529646
View Article PubMed/NCBIKarim FT, Sarker ZM, Ghafoor K, Al-Juhaimi FY, Jalil R, Awang MB, Amid M, Hossain MDS, Khalil HPSA. Microencapsulation of fish oil using hydroxypropyl methylcellulose as a carrier material by spray drying. J. Food Process. Preserv. 2016;40:140-153.
View ArticleNaik A, Meda V, Lele SS. Freeze drying for microencapsulation of α-linolenic acid rich oil: A functional ingredient from Lepidium sativum seeds. Eur. J. Lipid Sci. Technol. 2014;116:837-846.
View ArticleYazicioglu B, Sahin S, Sumnu G. Microencapsulation of wheat germ oil. J. Food Sci. Technol. 2015;52:3590-3597. PMid:26028741
View Article PubMed/NCBIAndrade MA, Cardoso MG, Andrade J, Silva LF, Teixeira ML, Resende JMV, Figueiredo AC, Barroso JG. Chemical composition and oxidant activity of essential oils from Cinnamodendron dinisii Schwacke and Siparuna guianensis Aublet. Antioxidants. 2013;2:384-397. PMid:26784471
View Article PubMed/NCBIKulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004;85:633-640.
View ArticleWang W, Wu N, Zu YG, Fu YJ. Antioxidant activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008;108:1019-1022. PMid:26065766
View Article PubMed/NCBIBeirao da Costa S, Duarte C, Bourbon AI, Pinheiro AC, Serra AT, Martins MM, Januario MIN, Vicente AA, Delgadillo I, Duarte C, Beirao da Costa ML. Effect of the matrix system in the delivery and in vitro bioactivity of microencapsulated oregano essential oil. J. Food Eng. 2012;110:190-199.
View ArticleNastruzzi C. Dextran cross-link gelatin microspheres as a drug delivery system. Eur. J. Pharm. Biopharm. 1999;47:153-160. 00076-9
View ArticleYin W, Yates MZ. Encapsulation and sustained release from biodegradable microcapsules made by emulsification/freeze drying and spray/freeze drying. J. Colloid Interface Sci. 2009;336:155-161. PMid:19423128
View Article PubMed/NCBIZhang Z, Grijpma DW, Feijen J. Poly(trimethylene carbonate) and monomethoxy poly(ethylene glycol) - block - poly(trimethylene carbonate) nanoparticles for the controlled release of dexamethasone. J. Control. Release. 2006;111:263-270. PMid:16481063
View Article PubMed/NCBIPrata AS, Zanin MHA, Re MI, Grosso CRF. Release properties of chemical and enzymatic crosslinked gelatin-gum Arabic microparticles containing a fluorescent probe plus vetiver essential oil. Colloids Surf., B. 2008;67:171-178. PMid:18835139
View Article PubMed/NCBIFernandes RVB, Borges SV, Botrel DA. Influence of spray drying operating conditions on microencapsulated rosemary oil properties. Ciênc. Tecnol. Aliment. 2013;33:171-178.
View ArticleOrtega-Rivas E. Characterization and processing relevance of food particulate materials. Part. Part. Syst. Char. 2012;29:192-203.
View ArticleQuispe-Condori S, Saldana DA, Temelli F. Microencapsulation of flax oil with zein using spray and freeze drying. LWT-Food Sci. Technol. 2011;44:1880-1887.
View ArticleSomboonvechakarn C, Barringer SA. Effect of particle density and composition on mixtures during nonelectrostatic and electrostatic powder coating. J. Food Process Eng. 2012;35:236-249.
View ArticleDhanalakshmi K, Ghosal S, Bhattachaya S. Agglomeration of food powder and applications. Crit. Rev. Food Sci. Nutr. 2011;51:432-441. PMid:21491268
View Article PubMed/NCBIShenoy P, Viau M, Tammel K, Innings F, Fitzpatrick J, Ahrne L. Effect of powder densities, particle size and shape on mixture quality of binary food powder mixtures. Powder Technol. 2015;272:165-172.
View ArticleBeristain CI, Garcia HS, Vernon-Carter EJ. Spray-dried encapsulation of cardamom (Elettaria cardamomum) essential oil with mesquite (Prosopis juliflora) gum. LWT-Food Sci. Technol. 2001;34:398-401.
View ArticleMoreau DL, Rosenberg M. Porosity of whey protein-based microcapsules containing anhydrous milkfat measured by gas displacement pycnometry. J. Food Sci. 1998;63:819-823.
View ArticleKrokida MK, Maroulis ZB. Effect of drying method on shrinkage and porosity. Dry. Technol. 1997;15:2441-2458.
View ArticleTurasan H, Sahin S, Sumnu G. Encapsulation of rosemary essential oil. LWT-Food Sci. Technol. 2015;64:112-119.
View ArticleAkhta M, Dickinson E. Whey protein-maltodextrin conjugates as emulsifying agents: An alternative to gum Arabic. Food Hydrocoll. 2007;21:607-616.
View ArticleKagami Y, Sugimura S, Fujishima N, Matsuda K, Kometani T, Matsumura Y. Oxidative stability, structure, and physical characteristics of microcapsules formed by spray drying of fish oil with protein and dextrin wall materials. J. Food Sci. 2003;68:2248-2255.
View ArticleBaranauskiene R, Venskutonis PR, Dewettinck K, Verhe R. Properties of oregano (Oreganum vulgare L.), citronella (Cymbopogon nardus G.) and marjoram (Majorana hortensis L.) flavors encapsulated into milk protein-based matrices. Food Res. Int. 2005;39:413-425.
View ArticleMikami I, Yamaguchi M, Shinmoto H, Tsuchida T. Development and validation of a microplate-based beta-carotene bleaching assay and comparison of antioxidant activity (AOA) in several crops measured by beta-carotene bleaching, DPPH and ORAC assays. Food Sci. Technol. Res. 2009;15;171-178.
View ArticleNigg J, Strobel G, Knighton WB, Hilmer J. Geary B, Riyaz-UI-Hassan S, Harper JK, Valenti D, Wang Y. Functionalized para-substituted benzenes as 1,8-cineole production modulators in an endophytic Nodulisporium species. Microbiol. 2014;160:1772-1782. PMid:24836622
View Article PubMed/NCBIBousbia N, Vian MA, Ferhat MA, Petitcolas E, Meklati BY, Chemat F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. J. Food Chem. 2009;114:355-362.
View ArticleWei A, Shibamoti T. Antioxidant/lipoxygenase inhibitory activities and chemical compositions of selected essential oils. J. Agr. Food Che,. 2010;58:7218-7225. PMid:20499917
View Article PubMed/NCBISchultze W, Alrehaily AJ, Mothana RA. Chemical analysis and biological activity of the essential oils of two endemic Soqotri Commiphora species. Mol. 2010;15:689-698. PMid:20335939
View Article PubMed/NCBIViuda-Martos M, Navajas YR, Zapata ES, Fernandez-Lopez J, Perez-Alvarez JA. Flav. Fragr. J. 2010;25:13-19.
View ArticleGourine N, Yousfi M, Bombarda I, Nadjemi B, Stocker P, Gaydou EM. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Ind. Crop. Prod. 2010;31:203-208.
View ArticleEdward-Jones V, Buck R, Shawcross SG, Dawson MM, Dunn K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns. 2004;30:772-777. PMid:15555788
View Article PubMed/NCBISiepmann J, Siepmann F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008;364:328-343. PMid:18822362
View Article PubMed/NCBIMaderuelo C, Zarzuelo A, Lanao JM. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release. 2011;154:2-19. PMid:21497624
View Article PubMed/NCBIThakhiew W, Waisayawan P, Devahastin S. Comparative evaluation of mathematical models for release of antioxidant from chitosan films prepared by different drying methods. Dry. Technol. 2011;29:1396-1403.
View ArticleWang TH, Hsia SM, Wu CH, Ko SY, Chen YH, Shieh TM, Chuang LC, Wu CY. Evaluation of the antibacterial potential oil liquid and vapor phase phenolic essential oil compounds against oral microorganisms. Plos One. 2016;11:e0163147. PMid:27681039
View Article PubMed/NCBIRiddick JA, Bunger WB, Sakano TK. Techniques of chemistry, in Organic Solvents. New York, NY: John Wiley and Sons, 1985.
Barceloux DG. Medical toxicology of natural substances: foods, fungi, medicinal herbs, plants, and venomous animals. Hoboken, NJ: John Wiley and Sons, 2008.
View ArticleGudi G, Krahmer A, Kruger H, Schulz H. ATR-FTIR spectroscopy on intact dried leaves of sage (Salvia officinalis L.) - Accelerated chemotaxonomic discrimination and analysis of essential oil composition. J. Agr. Food Chem. 2015;63:8743-8750. PMid:26360136
View Article PubMed/NCBINowak A, Kalemba D, Piotrowska M, Czyzowska A. Effects of thyme (Thymus vulgaris L.) and rosemary (Rosmarinus officinalis L.) essential oils on growth of Brochothrix thermosphacta. Afric. J. Microbiol. Res. 2013;7:3396-3404.
View ArticleKong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007;39:549-559. PMid:17687489
View Article PubMed/NCBITajmir-Riahi HA. An overview of drug binding to human serum albumin: protein folding and unfolding. Sci. Iran. 2007;14:87-95.
Forrest SA, Yada RY, Rousseau D. Interactions of vitamin D3 with bovine beta-lactoglobulin and beta-casein. J. Agr. Food Chem. 2005;53:8003-8009. PMid:16190663
View Article PubMed/NCBI